|
Isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable, organic compound with a pungent alcoholic odor. Isopropyl alcohol, an organic polar molecule, is miscible in water, ethanol, and chloroform, demonstrating its ability to dissolve a wide range of substances including ethyl cellulose, polyvinyl butyral, oils, alkaloids, and natural resins. Notably, it is not miscible with salt solutions and can be separated by adding sodium chloride in a process known as salting out. It forms an azeotrope with water, resulting in a boiling point of 80.37 °C and is characterized by its slightly bitter taste. Isopropyl alcohol becomes viscous at lower temperatures, freezing at −89.5 °C, and has significant ultraviolet-visible absorbance at 205 nm. Chemically, it can be oxidized to acetone or undergo various reactions to form compounds like isopropoxides or aluminium isopropoxide. As an isopropyl group linked ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubbing Alcohol
Rubbing alcohol, known as surgical spirit in the British Pharmacopoeia, refers to a group of denatured alcohol solutions commonly used as topical disinfectant. In addition to its medical applications, rubbing alcohol is employed in various industrial and household contexts. These solutions are primarily composed of either isopropyl alcohol (isopropanol) or ethanol, with isopropyl alcohol being the more widely available formulation. The United States Pharmacopeia (USP) defines "isopropyl rubbing alcohol USP" as a solution containing approximately 70% alcohol by volume of pure isopropanol, while "rubbing alcohol USP" refers to a solution containing approximately 70% by volume of denatured ethanol. In Ireland and the United Kingdom, the comparable product is "surgical spirit B.P.", defined by the British Pharmacopoeia as containing 95% methylated spirit, 2.5% castor oil, 2% diethyl phthalate, and 0.5% methyl salicylate. Known alternatively as "wintergreen oil", methyl salicylate is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
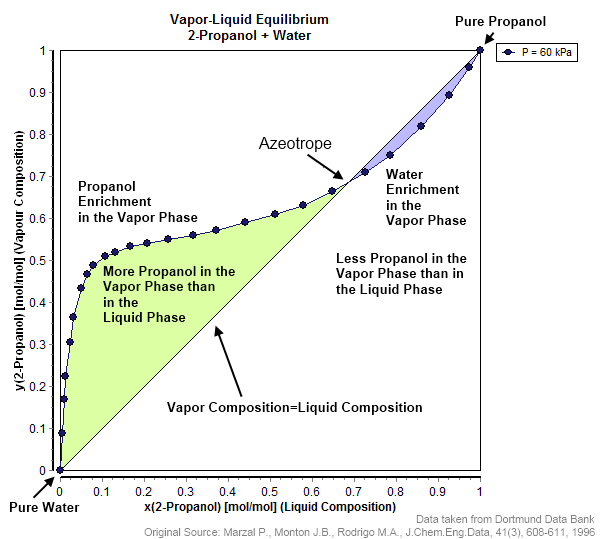
Azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Knowing an azeotrope's behavior is important for distillation. Each azeotrope has a characteristic boiling point. The boiling point of an azeotrope is either less than the boiling point temperatures of any of its constituents (a positive azeotrope), or greater than the boiling point of any of its constituents (a negative azeotrope). For both positive and negative azeotropes, it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. For technical applications, the pressure-temperature-composition behavior of a mixture is the most important, but other important ther ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
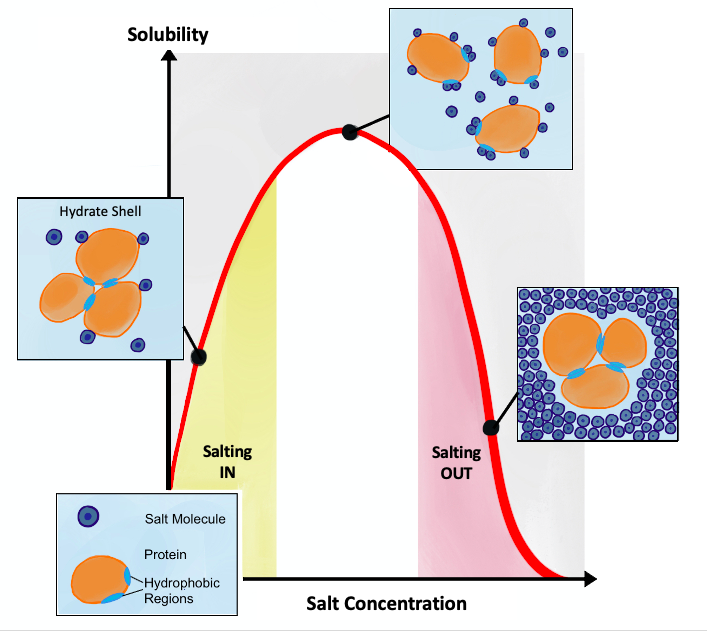
Salting Out
Salting out (also known as salt-induced precipitation, salt fractionation, anti-solvent crystallization, precipitation crystallization, or drowning out) is a purification technique that utilizes the reduced solubility of certain molecules in a solution of very high ionic strength. Salting out is typically used to precipitate large biomolecules, such as proteins or DNA. Because the salt concentration needed for a given protein to precipitate out of the solution differs from protein to protein, a specific salt concentration can be used to precipitate a target protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed. Principle Salt compounds dissociate in aqueous solutions. This property is exploited in the process of salting out. When the salt concentration is increased, some of the water molecules are attracted by the salt ions, which decreases the number of water molecules available to interact with the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs as the mineral halite. In its edible form, it is commonly used as a condiment and curing (food preservation), food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further Chemical synthesis, chemical syntheses. Another major application of sodium chloride is deicing of roadways in sub-freezing weather. Uses In addition to the many familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 data) include chemicals and de-icing.Westphal, Gisbert ''et al.'' (2002) "Sodium Chloride" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim . Chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resin
A resin is a solid or highly viscous liquid that can be converted into a polymer. Resins may be biological or synthetic in origin, but are typically harvested from plants. Resins are mixtures of organic compounds, predominantly terpenes. Common resins include amber, hashish, frankincense, myrrh and the animal-derived resin, shellac. Resins are used in varnishes, adhesives, food additives, incenses and perfumes. Resins protect plants from insects and pathogens, and are secreted in response to injury. Resins repel herbivores, insects, and pathogens, while the volatile natural phenol, phenolic compounds may attract benefactors such as predators of insects that attack the plant. Composition Most plant resins are composed of terpenes. Specific components are alpha-Pinene, alpha-pinene, pinene, beta-pinene, carene, delta-3 carene, and sabinene, the monocyclic terpenes limonene and terpinolene, and smaller amounts of the tricyclic sesquiterpenes, longifolene, caryophyllene, and cad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloids
Alkaloids are a broad class of naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids. Alkaloids are produced by a large variety of organisms including bacteria, fungi, plants, and animals. They can be purified from crude extracts of these organisms by acid-base extraction, or solvent extractions followed by silica-gel column chromatography. Alkaloids have a wide range of pharmacological activities including antimalarial (e.g. quinine), antiasthma (e.g. ephedrine), anticancer (e.g. homoharringtonine), cholinomimetic (e.g. galantamine), vasodilatory (e.g. vincamine), antiarrhythmic (e.g. quinidine), analgesic (e.g. morphine), antibacterial (e.g. chelerythrine), and antihyperglycemic activities (e.g. berberine). Many have found use in traditional or modern medicine, or as starting points for drug discovery. Other alkaloids possess psychotropic (e.g. psilocin) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyvinyl Butyral
Polyvinyl butyral (or PVB) is a resin mostly used for applications that require strong binding, optical clarity, adhesion to many surfaces, toughness and flexibility. It is prepared from polyvinyl alcohol by reaction with butyraldehyde. The major application is laminated safety glass for automobile windshields. Trade names for PVB-films include KB PVB, GUTMANN PVB, Saflex, GlasNovations, Butacite, WINLITE, S-Lec, Trosifol and EVERLAM. PVB is also available as 3D printer filament that is stronger and more heat resistant than polylactic acid (PLA). Applications Automotive and architectural Laminated glass, commonly used in the automotive and architectural fields, comprises a protective interlayer, usually polyvinyl butyral, bonded between two panels of glass. The bonding process takes place under heat and pressure. When laminated under these conditions, the PVB interlayer becomes optically clear and binds the two panes of glass together. Once sealed together, the glass "san ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Cellulose
Ethyl cellulose (or ethylcellulose) is a derivative of cellulose in which some of the hydroxyl groups on the repeating glucose units are converted into ethyl ether groups. The number of ethyl groups can vary depending on the manufacturer. It is mainly used as a thin-film coating material for coating paper, vitamin and medical pills, and for thickeners in cosmetics and in industrial processes. Food grade ethyl cellulose is one of few non-toxic films and thickeners which are not water-soluble. This property allows it to be used to safeguard ingredients from water. Ethyl cellulose is also used as a food additive as an emulsifier An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Althou ... (E462). Ethyl cellulose is commonly used as a coating material for tablets and capsules, as it provide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). Such substances are said to be miscible (etymologically equivalent to the common term " mixable"). The term is most often applied to liquids but also applies to solids and gases. An example in liquids is the miscibility of water and ethanol as they mix in all proportions. By contrast, substances are said to be immiscible if the mixture does not form a solution for certain proportions. For one example, oil is not soluble in water, so these two solvents are immiscible. As another example, butanone (methyl ethyl ketone) is immiscible in water: it is soluble in water up to about 275 grams per liter, but will separate into two phases beyond that. Organic compounds In organic compounds, the weight percent of hydrocarbon chain often determines the compound's miscibility with water. For examp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |