Biochemical Journal Front Cover on:
[Wikipedia]
[Google]
[Amazon]
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both
 It was once generally believed that life and its materials had some essential property or substance (often referred to as the "
It was once generally believed that life and its materials had some essential property or substance (often referred to as the "
 Lipids comprise a diverse range of
Lipids comprise a diverse range of
 Proteins are very large molecules—macro-biopolymers—made from monomers called amino acids. An amino acid consists of an alpha carbon atom attached to an amino group, –NH2, a
Proteins are very large molecules—macro-biopolymers—made from monomers called amino acids. An amino acid consists of an alpha carbon atom attached to an amino group, –NH2, a 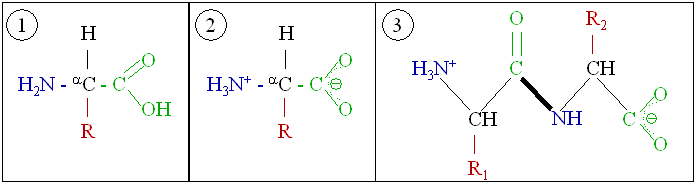
 Proteins can have structural and/or functional roles. For instance, movements of the proteins actin and
Proteins can have structural and/or functional roles. For instance, movements of the proteins actin and 
 Ingested proteins are usually broken up into single amino acids or dipeptides in the
Ingested proteins are usually broken up into single amino acids or dipeptides in the
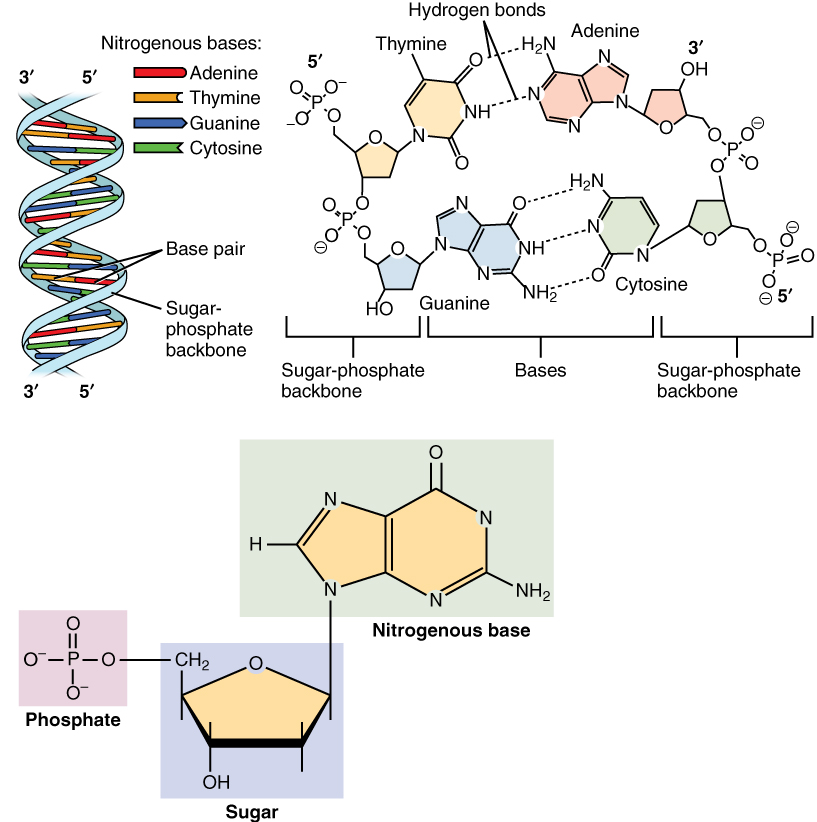 Nucleic acids, so-called because of their prevalence in cellular nuclei, is the generic name of the family of biopolymers. They are complex, high-molecular-weight biochemical macromolecules that can convey genetic information in all living cells and viruses. The monomers are called nucleotides, and each consists of three components: a nitrogenous heterocyclic base (either a purine or a
Nucleic acids, so-called because of their prevalence in cellular nuclei, is the generic name of the family of biopolymers. They are complex, high-molecular-weight biochemical macromolecules that can convey genetic information in all living cells and viruses. The monomers are called nucleotides, and each consists of three components: a nitrogenous heterocyclic base (either a purine or a  The most common nucleic acids are deoxyribonucleic acid (DNA) and
The most common nucleic acids are deoxyribonucleic acid (DNA) and
The Virtual Library of Biochemistry, Molecular Biology and Cell Biology
Biochemistry, 5th ed.
Full text of Berg, Tymoczko, and Stryer, courtesy of NCBI.
SystemsX.ch – The Swiss Initiative in Systems Biology
Full text of Biochemistry
by Kevin and Indira, an introductory biochemistry textbook. {{Authority control Biotechnology Molecular biology
chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
and biology, biochemistry may be divided into three fields: structural biology, enzymology
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all areas of the life sciences are being uncovered and developed through biochemical methodology and research.Voet Voet is the surname of:
* Alexander Voet the Elder (1608-1689), Flemish printmaker and publisher
*Alexander Voet the Younger (1637–1693/1705), Flemish printmaker and publisher
*Donald Voet, American biochemist and textbook author
* Gijsbert Voet ...
(2005), p. 3. Biochemistry focuses on understanding the chemical basis which allows biological molecules to give rise to the processes that occur within living cells
Cell most often refers to:
* Cell (biology), the functional basic unit of life
Cell may also refer to:
Locations
* Monastic cell, a small room, hut, or cave in which a religious recluse lives, alternatively the small precursor of a monastery w ...
and between cells, Karp (2009), p. 2. in turn relating greatly to the understanding of tissues and organs, as well as organism structure and function. Miller (2012). p. 62. Biochemistry is closely related to molecular biology, which is the study of the molecular
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
mechanisms of biological phenomena. Astbury (1961), p. 1124.
Much of biochemistry deals with the structures, bonding, functions, and interactions of biological macromolecule
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The ...
s, such as proteins, nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s, carbohydrates, and lipids. They provide the structure of cells and perform many of the functions associated with life. Eldra (2007), p. 45. The chemistry of the cell also depends upon the reactions of small molecules and ions. These can be inorganic (for example, water and metal ions) or organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
(for example, the amino acids, which are used to synthesize proteins). Marks (2012), Chapter 14. The mechanisms used by cells to harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition and agriculture. In medicine, biochemist
Biochemists are scientists who are trained in biochemistry. They study chemical processes and chemical transformations in living organisms. Biochemists study DNA, proteins and Cell (biology), cell parts. The word "biochemist" is a portmanteau of ...
s investigate the causes and cures of diseases. Nutrition studies how to maintain health and wellness and also the effects of nutritional deficiencies. UNICEF (2010), pp. 61, 75. In agriculture, biochemists investigate soil and fertilizers. Improving crop cultivation, crop storage, and pest control are also goals. Biochemistry is extremely important since it helps individuals learn about complicated topics such as prion
Prions are misfolded proteins that have the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. It ...
s.
History
At its most comprehensive definition, biochemistry can be seen as a study of the components and composition of living things and how they come together to become life. In this sense, the history of biochemistry may therefore go back as far as the ancient Greeks. Helvoort (2000), p. 81. However, biochemistry as a specific scientific discipline began sometime in the 19th century, or a little earlier, depending on which aspect of biochemistry is being focused on. Some argued that the beginning of biochemistry may have been the discovery of the first enzyme, diastase (now called amylase), in 1833 by Anselme Payen, while others considered Eduard Buchner's first demonstration of a complex biochemical process alcoholic fermentation in cell-free extracts in 1897 to be the birth of biochemistry. Some might also point as its beginning to the influential 1842 work byJustus von Liebig
Justus Freiherr von Liebig (12 May 1803 – 20 April 1873) was a German scientist who made major contributions to agricultural and biological chemistry, and is considered one of the principal founders of organic chemistry. As a professor at t ...
, ''Animal chemistry, or, Organic chemistry in its applications to physiology and pathology'', which presented a chemical theory of metabolism, or even earlier to the 18th century studies on fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food ...
and respiration by Antoine Lavoisier. Many other pioneers in the field who helped to uncover the layers of complexity of biochemistry have been proclaimed founders of modern biochemistry. Emil Fischer, who studied the chemistry of proteins, and F. Gowland Hopkins, who studied enzymes and the dynamic nature of biochemistry, represent two examples of early biochemists.
The term "biochemistry" itself is derived from a combination of biology and chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
. In 1877, Felix Hoppe-Seyler used the term (''biochemie'' in German) as a synonym for physiological chemistry in the foreword to the first issue of ''Zeitschrift für Physiologische Chemie
''Biological Chemistry'' is a peer-reviewed scientific journal focusing on biological chemistry. The journal is published by Walter de Gruyter and the current editor-in-chief is Bernhard Brüne.
History
The journal was established by Felix Ho ...
'' (Journal of Physiological Chemistry) where he argued for the setting up of institutes dedicated to this field of study. The German chemist Carl Neuberg however is often cited to have coined the word in 1903, Ben-Menahem (2009), p. 2982. while some credited it to Franz Hofmeister
Franz Hofmeister (30 August 1850, in Prague – 26 July 1922, in Würzburg) was an early protein scientist, and is famous for his studies of salts that influence the solubility and conformational stability of proteins. In 1902, Hofmeister became t ...
.
 It was once generally believed that life and its materials had some essential property or substance (often referred to as the "
It was once generally believed that life and its materials had some essential property or substance (often referred to as the "vital principle
Vitalism is a belief that starts from the premise that "living organisms are fundamentally different from non-living entities because they contain some non-physical element or are governed by different principles than are inanimate things." Wher ...
") distinct from any found in non-living matter, and it was thought that only living beings could produce the molecules of life. In 1828, Friedrich Wöhler
Friedrich Wöhler () FRS(For) HonFRSE (31 July 180023 September 1882) was a German chemist known for his work in inorganic chemistry, being the first to isolate the chemical elements beryllium and yttrium in pure metallic form. He was the firs ...
published a paper on his serendipitous urea synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
** Organic synthesis, the chemical synthesis of organ ...
from potassium cyanate and ammonium sulfate; some regarded that as a direct overthrow of vitalism and the establishment of organic chemistry.Kauffman
Kaufmann is a surname with many variants such as Kauffmann, Kaufman, and Kauffman. In German, the name means ''merchant''. It is the cognate of the English '' Chapman'' (which had a similar meaning in the Middle Ages, though it disappeared from ...
(2001), pp. 121–133. However, the Wöhler synthesis has sparked controversy as some reject the death of vitalism at his hands. Since then, biochemistry has advanced, especially since the mid-20th century, with the development of new techniques such as chromatography, X-ray diffraction
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
, dual polarisation interferometry, NMR spectroscopy, radioisotopic labeling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific ...
, electron microscopy
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a hi ...
and molecular dynamics simulations. These techniques allowed for the discovery and detailed analysis of many molecules and metabolic pathways of the cell, such as glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
and the Krebs cycle (citric acid cycle), and led to an understanding of biochemistry on a molecular level.
Another significant historic event in biochemistry is the discovery of the gene, and its role in the transfer of information in the cell. In the 1950s, James D. Watson, Francis Crick
Francis Harry Compton Crick (8 June 1916 – 28 July 2004) was an English molecular biologist, biophysicist, and neuroscientist. He, James Watson, Rosalind Franklin, and Maurice Wilkins played crucial roles in deciphering the helical struc ...
, Rosalind Franklin
Rosalind Elsie Franklin (25 July 192016 April 1958) was a British chemist and X-ray crystallographer whose work was central to the understanding of the molecular structures of DNA (deoxyribonucleic acid), RNA (ribonucleic acid), viruses, co ...
and Maurice Wilkins
Maurice Hugh Frederick Wilkins (15 December 1916 – 5 October 2004) was a New Zealand-born British biophysicist and Nobel laureate whose research spanned multiple areas of physics and biophysics, contributing to the scientific understanding o ...
were instrumental in solving DNA structure and suggesting its relationship with the genetic transfer of information. In 1958, George Beadle and Edward Tatum
Edward Lawrie Tatum (December 14, 1909 – November 5, 1975) was an American geneticist. He shared half of the Nobel Prize in Physiology or Medicine in 1958 with George Beadle for showing that genes control individual steps in metabolism. The o ...
received the Nobel Prize for work in fungi showing that one gene produces one enzyme. Krebs (2012), p. 32. In 1988, Colin Pitchfork was the first person convicted of murder with DNA evidence, which led to the growth of forensic science
Forensic science, also known as criminalistics, is the application of science to criminal and civil laws, mainly—on the criminal side—during criminal investigation, as governed by the legal standards of admissible evidence and criminal ...
.Butler
A butler is a person who works in a house serving and is a domestic worker in a large household. In great houses, the household is sometimes divided into departments with the butler in charge of the dining room, wine cellar, and pantry. Some a ...
(2009), p. 5. More recently, Andrew Z. Fire and Craig C. Mello
Craig Cameron Mello (born October 18, 1960) is an American biologist and professor of molecular medicine at the University of Massachusetts Medical School in Worcester, Massachusetts. He was awarded the 2006 Nobel Prize for Physiology or Medicine, ...
received the 2006 Nobel Prize for discovering the role of RNA interference (RNAi), in the silencing of gene expression
Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, protein or non-coding RNA, and ultimately affect a phenotype, as the final effect. The ...
. Chandan (2007), pp. 193–194.
Starting materials: the chemical elements of life
Around two dozenchemical elements
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
are essential to various kinds of biological life
Life is a quality that distinguishes matter that has biological processes, such as signaling and self-sustaining processes, from that which does not, and is defined by the capacity for growth, reaction to stimuli, metabolism, energy tran ...
. Most rare elements on Earth are not needed by life (exceptions being selenium and iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
), while a few common ones ( aluminum and titanium) are not used. Most organisms share element needs, but there are a few differences between plants and animals. For example, ocean algae use bromine, but land plants and animals do not seem to need any. All animals require sodium, but is not an essential element for plants. Plants need boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
and silicon, but animals may not (or may need ultra-small amounts).
Just six elements— carbon, hydrogen, nitrogen, oxygen, calcium and phosphorus—make up almost 99% of the mass of living cells, including those in the human body (see composition of the human body for a complete list). In addition to the six major elements that compose most of the human body, humans require smaller amounts of possibly 18 more.
Biomolecules
The 4 main classes of molecules in bio-chemistry (often calledbiomolecule
A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or development. Biomolecules include large ...
s) are carbohydrates, lipids, proteins, and nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s. Slabaugh (2007), pp. 3–6. Many biological molecules are polymers: in this terminology, monomers are relatively small macromolecules that are linked together to create large macromolecule
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The ...
s known as polymers. When monomers are linked together to synthesize a biological polymer, they undergo a process called dehydration synthesis. Different macromolecules can assemble in larger complexes, often needed for biological activity
In pharmacology, biological activity or pharmacological activity describes the beneficial or adverse effects of a drug on living matter. When a drug is a complex chemical mixture, this activity is exerted by the substance's active ingredient or ...
.
Carbohydrates
Two of the main functions of carbohydrates are energy storage and providing structure. One of the commonsugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double ...
s known as glucose is a carbohydrate, but not all carbohydrates are sugars. There are more carbohydrates on Earth than any other known type of biomolecule; they are used to store energy and genetic information, as well as play important roles in cell to cell interactions and communications.
The simplest type of carbohydrate is a monosaccharide
Monosaccharides (from Greek ''monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
They are usually colorless, water-solub ...
, which among other properties contains carbon, hydrogen, and oxygen, mostly in a ratio of 1:2:1 (generalized formula C''n''H2''n''O''n'', where ''n'' is at least 3). Glucose (C6H12O6) is one of the most important carbohydrates; others include fructose
Fructose, or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galacto ...
(C6H12O6), the sugar commonly associated with the sweet taste of fruits, Whiting (1970), pp. 1–31. and deoxyribose (C5H10O4), a component of DNA. A monosaccharide can switch between acyclic (open-chain) form and a cyclic form. The open-chain form can be turned into a ring of carbon atoms bridged by an oxygen atom created from the carbonyl group of one end and the hydroxyl group of another. The cyclic molecule has a hemiacetal or hemiketal
A hemiacetal or a hemiketal has the general formula R1R2C(OH)OR, where R1 or R2 is hydrogen or an organic substituent. They generally result from the addition of an alcohol to an aldehyde or a ketone, although the latter are sometimes called hemi ...
group, depending on whether the linear form was an aldose
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Aldoses can be distinguished from keto ...
or a ketose.
In these cyclic forms, the ring usually has 5 or 6 atoms. These forms are called furanoses and pyranose Pyranose is a collective term for saccharides that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. There may be other carbons external to the ring. The name derives from its similarity ...
s, respectively—by analogy with furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
and pyran, the simplest compounds with the same carbon-oxygen ring (although they lack the carbon-carbon double bonds of these two molecules). For example, the aldohexose glucose may form a hemiacetal linkage between the hydroxyl on carbon 1 and the oxygen on carbon 4, yielding a molecule with a 5-membered ring, called glucofuranose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
. The same reaction can take place between carbons 1 and 5 to form a molecule with a 6-membered ring, called glucopyranose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
. Cyclic forms with a 7-atom ring called heptoses
A heptose is a monosaccharide with seven carbon atoms.
They have either an aldehyde functional group in position 1 (aldoheptoses) or a ketone functional group in position 2, 3 or 4 (ketoheptoses). Ketoheptoses have 4 chiral centers, whereas aldoh ...
are rare.
Two monosaccharides can be joined by a glycosidic or ester bond
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
into a '' disaccharide'' through a dehydration reaction during which a molecule of water is released. The reverse reaction in which the glycosidic bond of a disaccharide is broken into two monosaccharides is termed '' hydrolysis''. The best-known disaccharide is sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refined ...
or ordinary sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double ...
, which consists of a glucose molecule and a fructose
Fructose, or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galacto ...
molecule joined. Another important disaccharide is lactose
Lactose is a disaccharide sugar synthesized by galactose and glucose subunits and has the molecular formula C12H22O11. Lactose makes up around 2–8% of milk (by mass). The name comes from ' (gen. '), the Latin word for milk, plus the suffix '' - ...
found in milk, consisting of a glucose molecule and a galactose molecule. Lactose may be hydrolysed by lactase, and deficiency in this enzyme results in lactose intolerance.
When a few (around three to six) monosaccharides are joined, it is called an '' oligosaccharide'' (''oligo-'' meaning "few"). These molecules tend to be used as markers and signals, as well as having some other uses. Varki (1999), p. 17. Many monosaccharides joined form a polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wa ...
. They can be joined in one long linear chain, or they may be branched. Two of the most common polysaccharides are cellulose and glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body.
Glycogen functions as one o ...
, both consisting of repeating glucose monomers. ''Cellulose'' is an important structural component of plant's cell wall
A cell wall is a structural layer surrounding some types of cells, just outside the cell membrane. It can be tough, flexible, and sometimes rigid. It provides the cell with both structural support and protection, and also acts as a filtering mech ...
s and ''glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body.
Glycogen functions as one o ...
'' is used as a form of energy storage in animals.
Sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double ...
can be characterized by having reducing or non-reducing ends. A reducing end
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reac ...
of a carbohydrate is a carbon atom that can be in equilibrium with the open-chain aldehyde (aldose
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Aldoses can be distinguished from keto ...
) or keto form ( ketose). If the joining of monomers takes place at such a carbon atom, the free hydroxy group of the pyranose Pyranose is a collective term for saccharides that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. There may be other carbons external to the ring. The name derives from its similarity ...
or furanose form is exchanged with an OH-side-chain of another sugar, yielding a full acetal. This prevents opening of the chain to the aldehyde or keto form and renders the modified residue non-reducing. Lactose contains a reducing end at its glucose moiety, whereas the galactose moiety forms a full acetal with the C4-OH group of glucose. Saccharose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refine ...
does not have a reducing end because of full acetal formation between the aldehyde carbon of glucose (C1) and the keto carbon of fructose (C2).
Lipids
 Lipids comprise a diverse range of
Lipids comprise a diverse range of molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
and to some extent is a catchall for relatively water-insoluble or nonpolar compounds of biological origin, including waxes, fatty acids, fatty-acid derived phospholipid
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s, sphingolipids, glycolipid
Glycolipids are lipids with a carbohydrate attached by a glycosidic (covalent) bond. Their role is to maintain the stability of the cell membrane and to facilitate cellular recognition, which is crucial to the immune response and in the connec ...
s, and terpenoids (e.g., retinoid
The retinoids are a class of chemical compounds that are vitamers of vitamin A or are chemically related to it. Retinoids have found use in medicine where they regulate epithelial cell growth.
Retinoids have many important functions throughout t ...
s and steroid
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and a ...
s). Some lipids are linear, open-chain aliphatic molecules, while others have ring structures. Some are aromatic (with a cyclic ingand planar latstructure) while others are not. Some are flexible, while others are rigid.
Lipids are usually made from one molecule of glycerol combined with other molecules. In triglycerides, the main group of bulk lipids, there is one molecule of glycerol and three fatty acids. Fatty acids are considered the monomer in that case, and may be saturated
Saturation, saturated, unsaturation or unsaturated may refer to:
Chemistry
* Saturation, a property of organic compounds referring to carbon-carbon bonds
** Saturated and unsaturated compounds
**Degree of unsaturation
** Saturated fat or fatty ac ...
(no double bonds in the carbon chain) or unsaturated (one or more double bonds in the carbon chain).
Most lipids have some polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
* Polar climate, the c ...
character in addition to being largely nonpolar. In general, the bulk of their structure is nonpolar or hydrophobic ("water-fearing"), meaning that it does not interact well with polar solvents like water. Another part of their structure is polar or hydrophilic ("water-loving") and will tend to associate with polar solvents like water. This makes them amphiphilic molecules (having both hydrophobic and hydrophilic portions). In the case of cholesterol, the polar group is a mere –OH (hydroxyl or alcohol). In the case of phospholipids, the polar groups are considerably larger and more polar, as described below.
Lipids are an integral part of our daily diet. Most oils and milk product
Dairy products or milk products, also known as lacticinia, are food products made from (or containing) milk. The most common dairy animals are cow, water buffalo, nanny goat, and ewe. Dairy products include common grocery store food items in th ...
s that we use for cooking and eating like butter, cheese
Cheese is a dairy product produced in wide ranges of flavors, textures, and forms by coagulation of the milk protein casein. It comprises proteins and fat from milk, usually the milk of cows, buffalo, goats, or sheep. During production, ...
, ghee
Ghee is a type of clarified butter, originating from India. It is commonly used in India for cooking, as a traditional medicine, and for religious rituals.
Description
Ghee is typically prepared by simmering butter, which is churned from c ...
etc., are composed of fats. Vegetable oils are rich in various polyunsaturated fatty acids (PUFA). Lipid-containing foods undergo digestion within the body and are broken into fatty acids and glycerol, which are the final degradation products of fats and lipids. Lipids, especially phospholipid
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s, are also used in various pharmaceutical product
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via insuffla ...
s, either as co-solubilisers (e.g., in parenteral infusions) or else as drug carrier
A drug carrier or drug vehicle is a substrate used in the process of drug delivery which serves to improve the selectivity, effectiveness, and/or safety of drug administration. Drug carriers are primarily used to control the release of drugs int ...
components (e.g., in a liposome or transfersome Transfersome is a proprietary drug delivery technology, an artificial vesicle
Vesicle may refer to:
; In cellular biology or chemistry
* Vesicle (biology and chemistry), a supramolecular assembly of lipid molecules, like a cell membrane
* Synapti ...
).
Proteins
carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
group, –COOH (although these exist as –NH3+ and –COO− under physiologic conditions), a simple hydrogen atom, and a side chain commonly denoted as "–R". The side chain "R" is different for each amino acid of which there are 20 standard ones. It is this "R" group that made each amino acid different, and the properties of the side-chains greatly influence the overall three-dimensional conformation of a protein. Some amino acids have functions by themselves or in a modified form; for instance, glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
functions as an important neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neuro ...
. Amino acids can be joined via a peptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein cha ...
. In this dehydration synthesis, a water molecule is removed and the peptide bond connects the nitrogen of one amino acid's amino group to the carbon of the other's carboxylic acid group. The resulting molecule is called a ''dipeptide A dipeptide is an organic compound derived from two amino acids. The constituent amino acids can be the same or different. When different, two isomers of the dipeptide are possible, depending on the sequence. Several dipeptides are physiologicall ...
'', and short stretches of amino acids (usually, fewer than thirty) are called ''peptides'' or polypeptides. Longer stretches merit the title ''proteins''. As an example, the important blood serum
Serum may refer to:
*Serum (blood), plasma from which the clotting proteins have been removed
**Antiserum, blood serum with specific antibodies for passive immunity
* Serous fluid, any clear bodily fluid
* Truth serum, a drug that is likely to mak ...
protein albumin contains 585 amino acid residues. Metzler (2001), p. 58.

 Proteins can have structural and/or functional roles. For instance, movements of the proteins actin and
Proteins can have structural and/or functional roles. For instance, movements of the proteins actin and myosin
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility.
The first myosin ...
ultimately are responsible for the contraction of skeletal muscle. One property many proteins have is that they specifically bind to a certain molecule or class of molecules—they may be ''extremely'' selective in what they bind. Antibodies
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the ...
are an example of proteins that attach to one specific type of molecule. Antibodies are composed of heavy and light chains. Two heavy chains would be linked to two light chains through disulfide linkages between their amino acids. Antibodies are specific through variation based on differences in the N-terminal domain.
The enzyme-linked immunosorbent assay (ELISA), which uses antibodies, is one of the most sensitive tests modern medicine uses to detect various biomolecules. Probably the most important proteins, however, are the enzymes. Virtually every reaction in a living cell requires an enzyme to lower the activation energy of the reaction. These molecules recognize specific reactant molecules called '' substrates''; they then catalyze the reaction between them. By lowering the activation energy, the enzyme speeds up that reaction by a rate of 1011 or more; a reaction that would normally take over 3,000 years to complete spontaneously might take less than a second with an enzyme. The enzyme itself is not used up in the process and is free to catalyze the same reaction with a new set of substrates. Using various modifiers, the activity of the enzyme can be regulated, enabling control of the biochemistry of the cell as a whole.
The structure of proteins is traditionally described in a hierarchy of four levels. The primary structure
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthes ...
of a protein consists of its linear sequence of amino acids; for instance, "alanine-glycine-tryptophan-serine-glutamate-asparagine-glycine-lysine-…". Secondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
is concerned with local morphology (morphology being the study of structure). Some combinations of amino acids will tend to curl up in a coil called an α-helix or into a sheet called a β-sheet; some α-helixes can be seen in the hemoglobin schematic above. Tertiary structure is the entire three-dimensional shape of the protein. This shape is determined by the sequence of amino acids. In fact, a single change can change the entire structure. The alpha chain of hemoglobin contains 146 amino acid residues; substitution of the glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
residue at position 6 with a valine residue changes the behavior of hemoglobin so much that it results in sickle-cell disease
Sickle cell disease (SCD) is a group of blood disorders typically inherited from a person's parents. The most common type is known as sickle cell anaemia. It results in an abnormality in the oxygen-carrying protein haemoglobin found in red blo ...
. Finally, quaternary structure is concerned with the structure of a protein with multiple peptide subunits, like hemoglobin with its four subunits. Not all proteins have more than one subunit.

 Ingested proteins are usually broken up into single amino acids or dipeptides in the
Ingested proteins are usually broken up into single amino acids or dipeptides in the small intestine
The small intestine or small bowel is an organ in the gastrointestinal tract where most of the absorption of nutrients from food takes place. It lies between the stomach and large intestine, and receives bile and pancreatic juice through the p ...
and then absorbed. They can then be joined to form new proteins. Intermediate products of glycolysis, the citric acid cycle, and the pentose phosphate pathway can be used to form all twenty amino acids, and most bacteria and plants possess all the necessary enzymes to synthesize them. Humans and other mammals, however, can synthesize only half of them. They cannot synthesize isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprot ...
, leucine, lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −C ...
, methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical ro ...
, phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the formula . It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino a ...
, threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO� ...
, tryptophan, and valine. Because they must be ingested, these are the essential amino acids. Mammals do possess the enzymes to synthesize alanine, asparagine, aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pro ...
, cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, sometime ...
, glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
, glutamine, glycine, proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the prot ...
, serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form un ...
, and tyrosine, the nonessential amino acids. While they can synthesize arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the am ...
and histidine, they cannot produce it in sufficient amounts for young, growing animals, and so these are often considered essential amino acids.
If the amino group is removed from an amino acid, it leaves behind a carbon skeleton called an α- keto acid. Enzymes called transaminases can easily transfer the amino group from one amino acid (making it an α-keto acid) to another α-keto acid (making it an amino acid). This is important in the biosynthesis of amino acids, as for many of the pathways, intermediates from other biochemical pathways are converted to the α-keto acid skeleton, and then an amino group is added, often via transamination. The amino acids may then be linked together to form a protein.
A similar process is used to break down proteins. It is first hydrolyzed into its component amino acids. Free ammonia (NH3), existing as the ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
ion (NH4+) in blood, is toxic to life forms. A suitable method for excreting it must therefore exist. Different tactics have evolved in different animals, depending on the animals' needs. Unicellular organisms release the ammonia into the environment. Likewise, bony fish
Osteichthyes (), popularly referred to as the bony fish, is a diverse superclass of fish that have skeletons primarily composed of bone tissue. They can be contrasted with the Chondrichthyes, which have skeletons primarily composed of cartilag ...
can release the ammonia into the water where it is quickly diluted. In general, mammals convert the ammonia into urea, via the urea cycle.
In order to determine whether two proteins are related, or in other words to decide whether they are homologous or not, scientists use sequence-comparison methods. Methods like sequence alignment
In bioinformatics, a sequence alignment is a way of arranging the sequences of DNA, RNA, or protein to identify regions of similarity that may be a consequence of functional, structural, or evolutionary relationships between the sequences. Alig ...
s and structural alignment
Structural alignment attempts to establish homology between two or more polymer structures based on their shape and three-dimensional conformation. This process is usually applied to protein tertiary structures but can also be used for large RN ...
s are powerful tools that help scientists identify homologies between related molecules. The relevance of finding homologies among proteins goes beyond forming an evolutionary pattern of protein families
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be ...
. By finding how similar two protein sequences are, we acquire knowledge about their structure and therefore their function.
Nucleic acids
 Nucleic acids, so-called because of their prevalence in cellular nuclei, is the generic name of the family of biopolymers. They are complex, high-molecular-weight biochemical macromolecules that can convey genetic information in all living cells and viruses. The monomers are called nucleotides, and each consists of three components: a nitrogenous heterocyclic base (either a purine or a
Nucleic acids, so-called because of their prevalence in cellular nuclei, is the generic name of the family of biopolymers. They are complex, high-molecular-weight biochemical macromolecules that can convey genetic information in all living cells and viruses. The monomers are called nucleotides, and each consists of three components: a nitrogenous heterocyclic base (either a purine or a pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other ...
), a pentose sugar, and a phosphate group.
ribonucleic acid
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
(RNA). The phosphate group and the sugar of each nucleotide bond with each other to form the backbone of the nucleic acid, while the sequence of nitrogenous bases stores the information. The most common nitrogenous bases are adenine, cytosine, guanine, thymine, and uracil. The nitrogenous bases of each strand of a nucleic acid will form hydrogen bonds
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
with certain other nitrogenous bases in a complementary strand of nucleic acid (similar to a zipper). Adenine binds with thymine and uracil, thymine binds only with adenine, and cytosine and guanine can bind only with one another. Adenine and Thymine & Adenine and Uracil contains two hydrogen Bonds, while Hydrogen Bonds formed between cytosine and guanine are three in number.
Aside from the genetic material of the cell, nucleic acids often play a role as second messenger
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. (Intercellular signals, a non-local form or cell signaling, encompassing both first me ...
s, as well as forming the base molecule for adenosine triphosphate (ATP), the primary energy-carrier molecule found in all living organisms. Also, the nitrogenous bases possible in the two nucleic acids are different: adenine, cytosine, and guanine occur in both RNA and DNA, while thymine occurs only in DNA and uracil occurs in RNA.
Metabolism
Carbohydrates as energy source
Glucose is an energy source in most life forms. For instance, polysaccharides are broken down into their monomers by enzymes ( glycogen phosphorylase removes glucose residues from glycogen, a polysaccharide). Disaccharides like lactose or sucrose are cleaved into their two component monosaccharides.Glycolysis (anaerobic)
Glucose is mainly metabolized by a very important ten-steppathway
Pathway or pathways may refer to:
Entertainment
* ''The Pathway'' (novel), a 1914 work by Gertrude Page
*''The Pathway'', a 2001 album by Officium Triste
* ''Pathway'' (album), by the Flaming Stars
* ''Pathways'' (album) (2010), by the Dave Hol ...
called glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
, the net result of which is to break down one molecule of glucose into two molecules of pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic aci ...
. This also produces a net two molecules of ATP
ATP may refer to:
Companies and organizations
* Association of Tennis Professionals, men's professional tennis governing body
* American Technical Publishers, employee-owned publishing company
* ', a Danish pension
* Armenia Tree Project, non ...
, the energy currency of cells, along with two reducing equivalents of converting NAD+ (nicotinamide adenine dinucleotide: oxidized form) to NADH (nicotinamide adenine dinucleotide: reduced form). This does not require oxygen; if no oxygen is available (or the cell cannot use oxygen), the NAD is restored by converting the pyruvate to lactate (lactic acid) (e.g., in humans) or to ethanol plus carbon dioxide (e.g., in yeast). Other monosaccharides like galactose and fructose can be converted into intermediates of the glycolytic pathway.
Aerobic
In aerobic cells with sufficient oxygen, as in most human cells, the pyruvate is further metabolized. It is irreversibly converted toacetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for ...
, giving off one carbon atom as the waste product carbon dioxide, generating another reducing equivalent as NADH. The two molecules acetyl-CoA (from one molecule of glucose) then enter the citric acid cycle, producing two molecules of ATP, six more NADH molecules and two reduced (ubi)quinones (via FADH2 as enzyme-bound cofactor), and releasing the remaining carbon atoms as carbon dioxide. The produced NADH and quinol molecules then feed into the enzyme complexes of the respiratory chain, an electron transport system transferring the electrons ultimately to oxygen and conserving the released energy in the form of a proton gradient over a membrane ( inner mitochondrial membrane in eukaryotes). Thus, oxygen is reduced to water and the original electron acceptors NAD+ and quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds
are regenerated. This is why humans breathe in oxygen and breathe out carbon dioxide. The energy released from transferring the electrons from high-energy states in NADH and quinol is conserved first as proton gradient and converted to ATP via ATP synthase. This generates an additional ''28'' molecules of ATP (24 from the 8 NADH + 4 from the 2 quinols), totaling to 32 molecules of ATP conserved per degraded glucose (two from glycolysis + two from the citrate cycle). It is clear that using oxygen to completely oxidize glucose provides an organism with far more energy than any oxygen-independent metabolic feature, and this is thought to be the reason why complex life appeared only after Earth's atmosphere accumulated large amounts of oxygen.
uch as benzene or naphthalene
Uch ( pa, ;
ur, ), frequently referred to as Uch Sharīf ( pa, ;
ur, ; ''"Noble Uch"''), is a historic city in the southern part of Pakistan's Punjab province. Uch may have been founded as Alexandria on the Indus, a town founded by Alexand ...
by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double ...Gluconeogenesis
In vertebrates, vigorously contractingskeletal muscle
Skeletal muscles (commonly referred to as muscles) are organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other types of muscl ...
s (during weightlifting or sprinting, for example) do not receive enough oxygen to meet the energy demand, and so they shift to anaerobic metabolism, converting glucose to lactate.
The combination of glucose from noncarbohydrates origin, such as fat and proteins. This only happens when glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body.
Glycogen functions as one o ...
supplies in the liver are worn out. The pathway is a crucial reversal of glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
from pyruvate to glucose and can use many sources like amino acids, glycerol and Krebs Cycle. Large scale protein and fat catabolism
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipids, ...
usually occur when those suffer from starvation or certain endocrine disorders. The liver regenerates the glucose, using a process called gluconeogenesis
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrat ...
. This process is not quite the opposite of glycolysis, and actually requires three times the amount of energy gained from glycolysis (six molecules of ATP are used, compared to the two gained in glycolysis). Analogous to the above reactions, the glucose produced can then undergo glycolysis in tissues that need energy, be stored as glycogen (or starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets ...
in plants), or be converted to other monosaccharides or joined into di- or oligosaccharides. The combined pathways of glycolysis during exercise, lactate's crossing via the bloodstream to the liver, subsequent gluconeogenesis and release of glucose into the bloodstream is called the Cori cycle
The Cori cycle (also known as the lactic acid cycle), named after its discoverers, Carl Ferdinand Cori and Gerty Cori, is a metabolic pathway in which lactate, produced by anaerobic glycolysis in muscles, is transported to the liver and converte ...
. Fromm and Hargrove (2012), pp. 183–194.
Relationship to other "molecular-scale" biological sciences
Researchers in biochemistry use specific techniques native to biochemistry, but increasingly combine these with techniques and ideas developed in the fields of genetics, molecular biology, and biophysics. There is not a defined line between these disciplines. Biochemistry studies thechemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
required for biological activity of molecules, molecular biology studies their biological activity, genetics studies their heredity, which happens to be carried by their genome. This is shown in the following schematic that depicts one possible view of the relationships between the fields:
* ''Biochemistry'' is the study of the chemical substances and vital processes occurring in live organisms. Biochemist
Biochemists are scientists who are trained in biochemistry. They study chemical processes and chemical transformations in living organisms. Biochemists study DNA, proteins and Cell (biology), cell parts. The word "biochemist" is a portmanteau of ...
s focus heavily on the role, function, and structure of biomolecule
A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or development. Biomolecules include large ...
s. The study of the chemistry behind biological processes and the synthesis of biologically active molecules are applications of biochemistry. Biochemistry studies life at the atomic and molecular level.
* ''Genetics'' is the study of the effect of genetic differences in organisms. This can often be inferred by the absence of a normal component (e.g. one gene). The study of " mutants" – organisms that lack one or more functional components with respect to the so-called " wild type" or normal phenotype. Genetic interactions (epistasis
Epistasis is a phenomenon in genetics in which the effect of a gene mutation is dependent on the presence or absence of mutations in one or more other genes, respectively termed modifier genes. In other words, the effect of the mutation is dep ...
) can often confound simple interpretations of such "knockout
A knockout (abbreviated to KO or K.O.) is a fight-ending, winning criterion in several full-contact combat sports, such as boxing, kickboxing, muay thai, mixed martial arts, karate, some forms of taekwondo and other sports involving striking, a ...
" studies.
* ''Molecular biology'' is the study of molecular underpinnings of the biological phenomena, focusing on molecular synthesis, modification, mechanisms and interactions. The central dogma of molecular biology
The central dogma of molecular biology is an explanation of the flow of genetic information within a biological system. It is often stated as "DNA makes RNA, and RNA makes protein", although this is not its original meaning. It was first stated by ...
, where genetic material is transcribed into RNA and then translated into protein, despite being oversimplified, still provides a good starting point for understanding the field. This concept has been revised in light of emerging novel roles for RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
.
* ''Chemical biology
Chemical biology is a scientific discipline spanning the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and ma ...
'' seeks to develop new tools based on small molecules that allow minimal perturbation of biological systems while providing detailed information about their function. Further, chemical biology employs biological systems to create non-natural hybrids between biomolecules and synthetic devices (for example emptied viral capsid
A capsid is the protein shell of a virus, enclosing its genetic material. It consists of several oligomeric (repeating) structural subunits made of protein called protomers. The observable 3-dimensional morphological subunits, which may or ma ...
s that can deliver gene therapy or drug molecules).
See also
Lists
* Important publications in biochemistry (chemistry) *List of biochemistry topics
A ''list'' is any set of items in a row. List or lists may also refer to:
People
* List (surname)
Organizations
* List College, an undergraduate division of the Jewish Theological Seminary of America
* SC Germania List, German rugby unio ...
* List of biochemists
This is a list of biochemists. It should include those who have been important to the development or practice of biochemistry. Their research or applications have made significant contributions in the area of basic or applied biochemistry.
{{co ...
* List of biomolecules
This is a list of articles that describe particular biomolecules or types of biomolecules.
A
For substances with an A- or α- prefix such as
α-amylase, please see the parent page (in this case Amylase).
* A23187 (Calcimycin, Calcium Ionopho ...
See also
*Astrobiology
Astrobiology, and the related field of exobiology, is an interdisciplinary scientific field that studies the origins, early evolution, distribution, and future of life in the universe. Astrobiology is the multidisciplinary field that investig ...
* Biochemistry (journal)
* Biological Chemistry (journal)
* Biophysics
* Chemical ecology
* Computational biomodeling Modelling biological systems is a significant task of systems biology and mathematical biology. Computational systems biology aims to develop and use efficient algorithms, data structures, visualization and communication tools with the goal of compu ...
* Dedicated bio-based chemical
Bioplastics are plastic materials produced from renewable biomass sources, such as vegetable fats and oils, corn starch, straw, woodchips, sawdust, recycled food waste, etc. Some bioplastics are obtained by processing directly from natural bio ...
* EC number
* Hypothetical types of biochemistry
* International Union of Biochemistry and Molecular Biology
* Metabolome
* Metabolomics
* Molecular biology
* Molecular medicine
* Plant biochemistry
Plant physiology is a subdiscipline of botany concerned with the functioning, or physiology, of plants. Closely related fields include plant morphology (structure of plants), plant ecology (interactions with the environment), phytochemistry (bio ...
* Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called protease ...
* Small molecule
* Structural biology
* TCA cycle
Notes
a. Fructose is not the only sugar found in fruits. Glucose and sucrose are also found in varying quantities in various fruits, and sometimes exceed the fructose present. For example, 32% of the edible portion of a date is glucose, compared with 24% fructose and 8% sucrose. However, peaches contain more sucrose (6.66%) than they do fructose (0.93%) or glucose (1.47%). Whiting, G.C. (1970), p. 5.References
Cited literature
* * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * *Further reading
* Fruton, Joseph S. ''Proteins, Enzymes, Genes: The Interplay of Chemistry and Biology''. Yale University Press: New Haven, 1999. * Keith Roberts, Martin Raff, Bruce Alberts, Peter Walter, Julian Lewis and Alexander Johnson, ''Molecular Biology of the Cell'' ** 4th Edition, Routledge, March, 2002, hardcover, 1616 pp. ** 3rd Edition, Garland, 1994, ** 2nd Edition, Garland, 1989, * Kohler, Robert. ''From Medical Chemistry to Biochemistry: The Making of a Biomedical Discipline''. Cambridge University Press, 1982. *External links
*The Virtual Library of Biochemistry, Molecular Biology and Cell Biology
Biochemistry, 5th ed.
Full text of Berg, Tymoczko, and Stryer, courtesy of NCBI.
SystemsX.ch – The Swiss Initiative in Systems Biology
Full text of Biochemistry
by Kevin and Indira, an introductory biochemistry textbook. {{Authority control Biotechnology Molecular biology