|
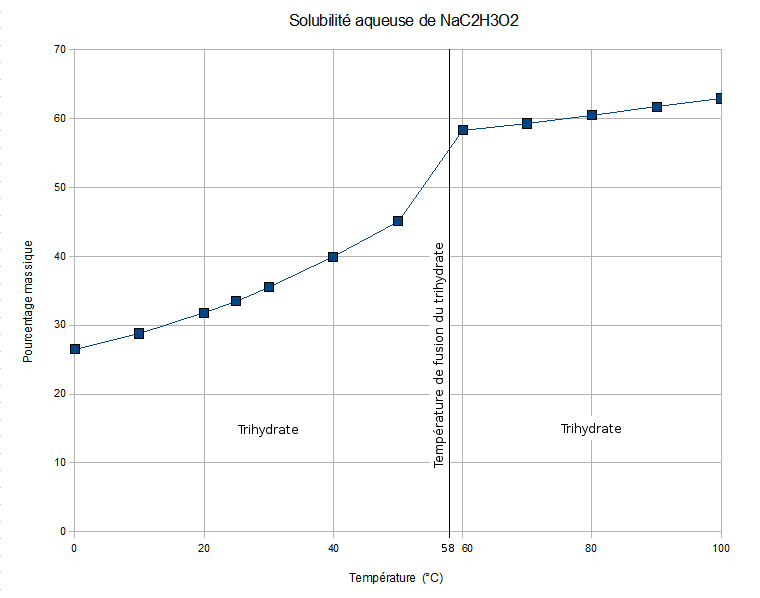
Sodium Acetate
Sodium acetate, CH3COONa, also abbreviated Sodium, NaOxygen, OAcetyl, Ac, is the sodium Salt (chemistry), salt of acetic acid. This salt is colorless, deliquescent, and hygroscopy, hygroscopic. Applications Biotechnological Sodium acetate is used as the carbon source (biology) , carbon source for culturing Bacterial growth, bacteria. Sodium acetate can also be useful for increasing yields of DNA extraction, DNA isolation by ethanol precipitation. Industrial Sodium acetate is used in the textile industry to neutralize sulfuric acid waste streams and also as a photoresist while using aniline dyes. It is also a pickling (metal), pickling agent in chrome Tanning (leather), tanning and helps to impede vulcanization of chloroprene in synthetic rubber production. It is also used to reduce static electricity during production of disposable cotton pads. Concrete longevity Sodium acetate is used as a sealant to mitigate water damage to concrete. It is environm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
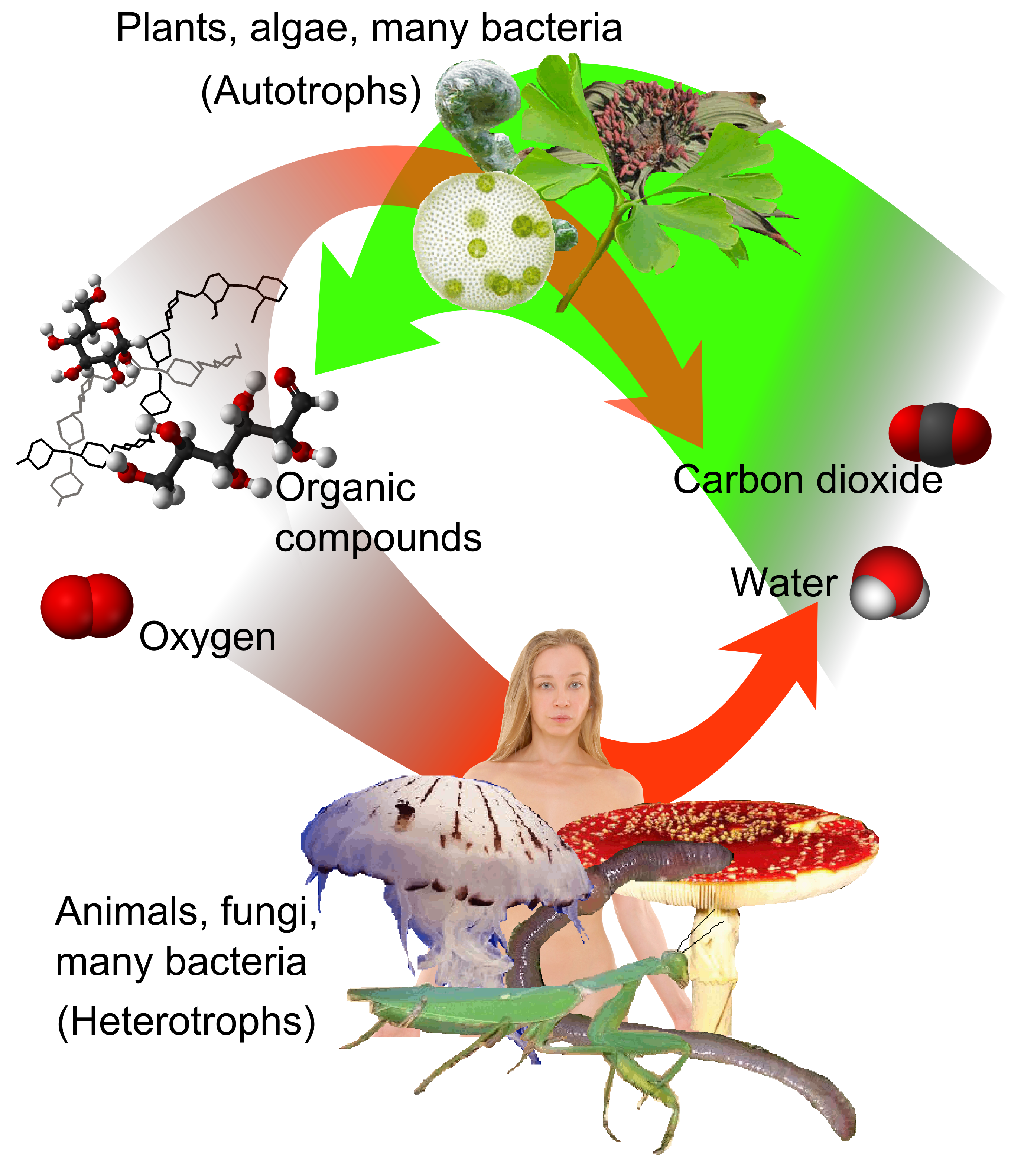
Carbon Source (biology)
A carbon source is a carbon-containing molecule that is used by an organism to synthesise biomass. Such sources may be organic or inorganic. Heterotrophs must use organic molecules as a source of both carbon and energy. In contrast, autotrophs may use inorganic materials as a source for both, such as inorganic chemical energy (chemolithotrophs) or light (photoautotrophs). The carbon cycle, which begins with an inorganic carbon source (such as carbon dioxide) and progresses through the biological carbon fixation process, includes the biological use of carbon as one of its components. /sup> Types of organism by carbon source Autotrophs Heterotrophs See also * Carbon-based life Carbon is a primary component of all known life on Earth, and represents approximately 45–50% of all dry biomass. Carbon compounds occur naturally in great abundance on Earth. Complex biological molecules consist of carbon atoms bonded with ot ... * References {{Reflist Carbon cycle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
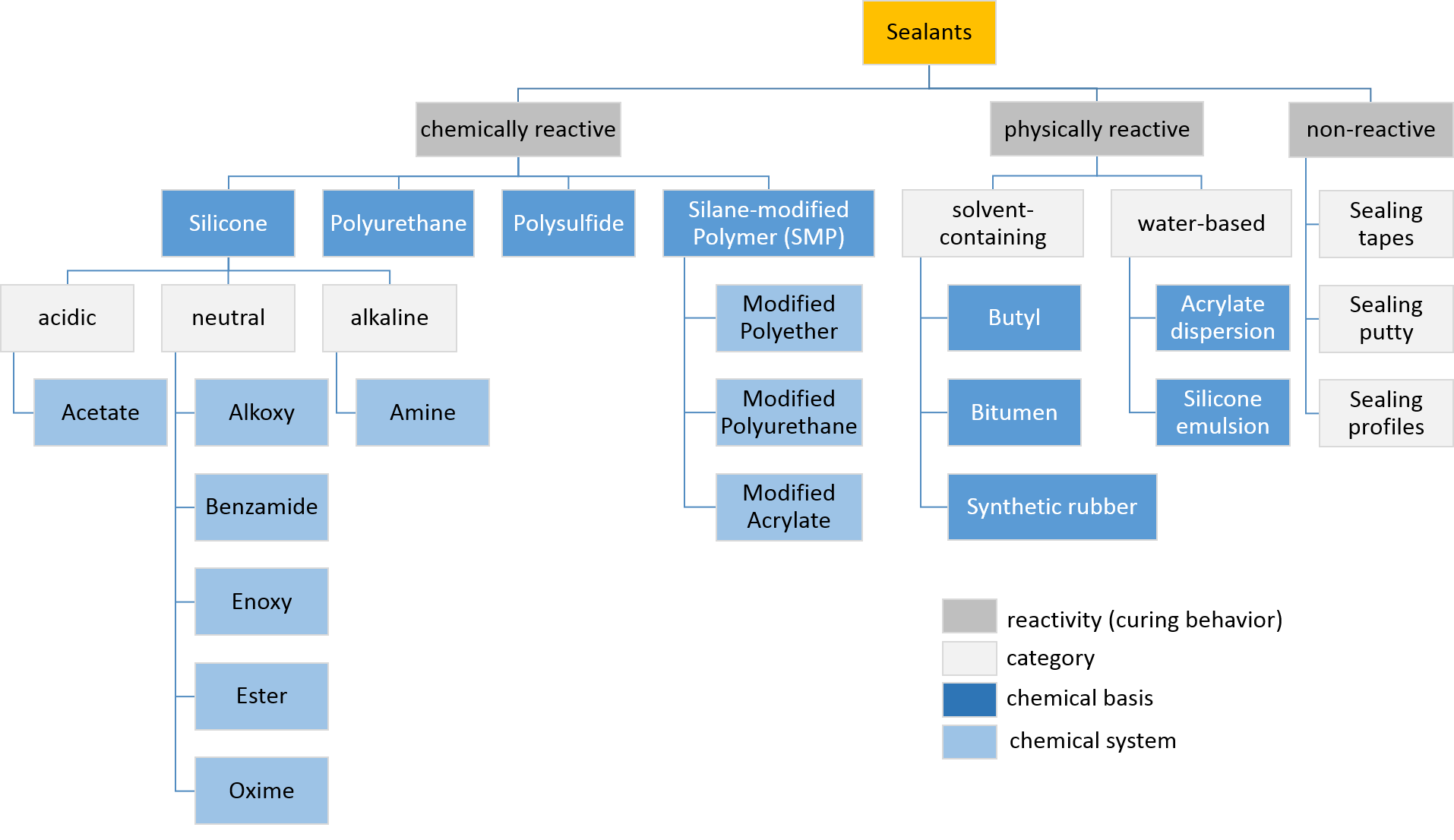
Sealant
Sealant is a substance used to block the passage of fluids through openings in materials, a type of Seal (mechanical), mechanical seal. In building construction ''sealant'' is sometimes synonymous with ''caulk'' (especially if acrylic latex or polyurethane based) and also serve the purposes of blocking dust, sound and heat transmission. Sealants may be weak or strong, flexible or rigid, permanent or temporary. Sealants are not adhesives but some have adhesive qualities and are called ''adhesive-sealants'' or ''structural sealants''. History Sealants were first used in prehistory in the broadest sense as mud, grass and reeds to seal dwellings from the weather such as the daub in wattle and daub and thatching. Natural sealants and adhesive-sealants included plant resins such as pine pitch and birch pitch, bitumen, wax, tar, natural gum, clay (mud) mortar, lime mortar, lead, blood and egg. In the 17th century glazing putty was first used to seal window glass made with linseed oil a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Static Electricity
Static electricity is an imbalance of electric charges within or on the surface of a material. The charge remains until it can move away by an electric current or electrical discharge. The word "static" is used to differentiate it from electric current, current electricity, where an electric charge flows through an electrical conductor. A static electric charge can be created whenever two surfaces contact and/or slide against each other and then separate. The effects of static electricity are familiar to most people because they can feel, hear, and even see sparks if the excess charge is neutralized when brought close to an electrical conductor (for example, a path to ground), or a region with an excess charge of the opposite polarity (positive or negative). The familiar phenomenon of a static shockmore specifically, an electrostatic dischargeis caused by the neutralization of a charge. Causes Materials are made of atoms that are normally electrically neutral because they contai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Synthetic Rubber
A synthetic rubber is an artificial elastomer. They are polymers synthesized from petroleum byproducts. About of rubber is produced annually in the United States, and of that amount two thirds are synthetic. Synthetic rubber, just like natural rubber, has many uses in the automotive industry for tires, door and window profiles, seals such as O-rings and gaskets, hoses, belts, matting, and flooring. They offer a different range of physical and chemical properties which can improve the reliability of a given product or application. Synthetic rubbers are superior to natural rubbers in two major respects: thermal stability, and resistance to oils and related compounds. They are more resistant to oxidizing agents, such as oxygen and ozone which can reduce the life of products like tires. History The expanded use of bicycles, and particularly their pneumatic tires, starting in the 1890s, created increased demand for rubber. In 1909, a team headed by Fritz Hofmann, working at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chloroprene
Chloroprene (IUPAC name 2-chlorobuta-1,3-diene) is a chemical compound with the molecular formula CH2=CCl−CH=CH2. Chloroprene is a colorless volatile liquid, almost exclusively used as a monomer for the production of the polymer polychloroprene, better known as neoprene, a type of synthetic rubber. History Although it may have been discovered earlier, chloroprene was largely developed by DuPont during the early 1930s, specifically with the formation of neoprene in mind. The chemists Elmer K. Bolton, Wallace Carothers, Arnold Collins and Ira Williams are generally accredited with its development and commercialisation although the work was based upon that of Julius Arthur Nieuwland, with whom they collaborated. Production Chloroprene is produced in three steps from 1,3-butadiene: (i) chlorination, (ii) isomerization of part of the product stream, and (iii) dehydrochlorination of 3,4-dichlorobut-1-ene. Chlorine adds to 1,3-butadiene to afford a mixture of 3,4-dichlorobut-1-e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Vulcanization
Vulcanization (British English: vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to include the hardening of other (synthetic) rubbers via various means. Examples include silicone rubber via room temperature vulcanizing and chloroprene rubber (neoprene) using metal oxides. Vulcanization can be defined as the curing of elastomers, with the terms 'vulcanization' and 'curing' sometimes used interchangeably in this context. It works by forming cross-links between sections of the polymer chain which results in increased rigidity and durability, as well as other changes in the mechanical and electrical properties of the material. Vulcanization, in common with the curing of other thermosetting polymers, is generally irreversible. The word was suggested by William Brockedon (a friend of Thomas Hancock who attained the B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tanning (leather)
Tanning, or hide tanning, is the process of treating Skinning, skins and Hide (skin), hides of animals to produce leather. A tannery is the place where the skins are processed. Historically, vegetable based tanning used tannin, an acidic chemical compound derived from the bark of certain trees, in the production of leather. An alternative method, developed in the 1800s, is chrome tanning, where chromium salts are used instead of natural tannins. History Tanning hide into leather involves a process which permanently alters the protein structure of skin, making it more durable and less susceptible to decomposition and coloring. The place where hides are processed is known as a ''tannery''. The English word for tanning is from the medieval Latin verb , from the noun (oak bark). This term may be derived from a Celtic word related to the Proto-Indo-European *' meaning 'fir tree'. (The same root is the source for Old High German meaning 'fir', related to modern German ''Tannenb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Pickling (metal)
Pickling is a metal surface treatment used to remove impurities, such as stains, inorganic contaminants, and rust or scale from ferrous metals, copper, precious metals and aluminium alloys. A solution called ''pickle liquor'', which usually contains acid, is used to remove the surface impurities. It is commonly used to descale or clean steel in various steelmaking processes. Process Metal surfaces can contain impurities that may affect usage of the product or further processing like plating with metal or painting. Various chemical solutions are usually used to clean these impurities. Strong acids, such as hydrochloric acid and sulfuric acid are common, but different applications use various other acids. Also alkaline solutions can be used for cleaning metal surfaces. Solutions usually also contain additives such as wetting agents and corrosion inhibitors. Pickling is sometimes called acid cleaning if descaling is not needed. Many hot working processes and other processes that o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Aniline Dyes
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Anili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronics industry. The process begins by coating a substrate with a light-sensitive organic material. A patterned mask is then applied to the surface to block light, so that only unmasked regions of the material will be exposed to light. A solvent, called a developer, is then applied to the surface. In the case of a positive photoresist, the photo-sensitive material is degraded by light and the developer will dissolve away the regions that were exposed to light, leaving behind a coating where the mask was placed. In the case of a negative photoresist, the photosensitive material is strengthened (either polymerized or cross-linked) by light, and the developer will dissolve away only the regions that were not exposed to light, leaving behind a coating in areas w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula . It is a colorless, odorless, and Viscosity, viscous liquid that is Miscibility, miscible with water. Pure sulfuric acid does not occur naturally due to its Dehydration reaction, strong affinity to water vapor; it is Hygroscopy, hygroscopic and readily absorbs water vapor from the Atmosphere of Earth, air. Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is releas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |