Sodium acetate on:
[Wikipedia]
[Google]
[Amazon]
Sodium acetate, CH3COONa, also abbreviated Na O Ac, is the
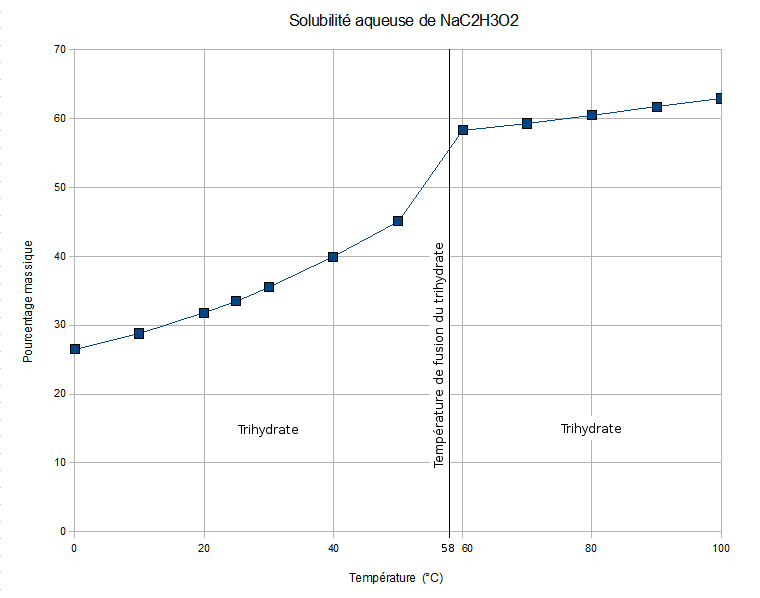 Sodium acetate trihydrate crystals melt at , and the liquid sodium acetate dissolves in the released
Sodium acetate trihydrate crystals melt at , and the liquid sodium acetate dissolves in the released
Thermal Energy Storage: Systems and Applications
page 155. Unlike some types of heat packs, such as those dependent upon irreversible chemical reactions, a sodium acetate heat pack can be easily reused by immersing the pack in boiling water for a few minutes, until the crystals are completely dissolved, and allowing the pack to slowly cool to room temperature.
Hot Ice – Instructions, Pictures, and VideosHow Sodium Acetate heating pads work
* {{Authority control Acetates E-number additives Food additives Organic sodium salts Photographic chemicals
sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
. This salt is colorless, deliquescent
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption (chemistry), absorption or adsorption from the surrounding Natural environment, environment, which is usually at normal or room temperature. If water mol ...
, and hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption (chemistry), absorption or adsorption from the surrounding Natural environment, environment, which is usually at normal or room temperature. If water mol ...
.
Applications
Biotechnological
Sodium acetate is used as the carbon source for culturingbacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
. Sodium acetate can also be useful for increasing yields of DNA isolation by ethanol precipitation.
Industrial
Sodium acetate is used in thetextile
Textile is an Hyponymy and hypernymy, umbrella term that includes various Fiber, fiber-based materials, including fibers, yarns, Staple (textiles)#Filament fiber, filaments, Thread (yarn), threads, and different types of #Fabric, fabric. ...
industry to neutralize sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
waste streams and also as a photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronics industry.
T ...
while using aniline dyes. It is also a pickling
Pickling is the process of food preservation, preserving or extending the shelf life of food by either Anaerobic organism, anaerobic fermentation (food), fermentation in brine or immersion in vinegar. The pickling procedure typically affects t ...
agent in chrome tanning and helps to impede vulcanization
Vulcanization (British English: vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to ...
of chloroprene in synthetic rubber
A synthetic rubber is an artificial elastomer. They are polymers synthesized from petroleum byproducts. About of rubber is produced annually in the United States, and of that amount two thirds are synthetic. Synthetic rubber, just like natural ru ...
production. It is also used to reduce static electricity
Static electricity is an imbalance of electric charges within or on the surface of a material. The charge remains until it can move away by an electric current or electrical discharge. The word "static" is used to differentiate it from electric ...
during production of disposable cotton pads.
Concrete longevity
Sodium acetate is used as asealant
Sealant is a substance used to block the passage of fluids through openings in materials, a type of Seal (mechanical), mechanical seal. In building construction ''sealant'' is sometimes synonymous with ''caulk'' (especially if acrylic latex or ...
to mitigate water damage to concrete
Concrete is a composite material composed of aggregate bound together with a fluid cement that cures to a solid over time. It is the second-most-used substance (after water), the most–widely used building material, and the most-manufactur ...
. It is environmentally benign and cheaper than the commonly used epoxy
Epoxy is the family of basic components or Curing (chemistry), cured end products of epoxy Resin, resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide fun ...
alternative for sealing concrete against water permeation
In physics and engineering, permeation (also called imbuing) is the penetration of a wikt:permeate#English, permeate (a fluid such as a liquid, gas, or vapor) through a solid. It is directly related to the concentration gradient of the permeate, ...
.
Food
Sodium acetate (anhydrous) is widely used as a shelf-life extending agent and pH control agent . It is safe to eat at low concentration.Buffer solution
A solution of sodium acetate (a basic salt of acetic acid) and acetic acid can act as a buffer to keep a relatively constant pH level. This is useful especially in biochemical applications where reactions are pH-dependent in a mildly acidic range (pH 4–6).Heating pad
Sodium acetate is also used in heating pads, hand warmers, and "hot ice". Asupersaturated
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a ...
solution of sodium acetate in water is supplied with a device to initiate crystallization, a process that releases substantial heat.
 Sodium acetate trihydrate crystals melt at , and the liquid sodium acetate dissolves in the released
Sodium acetate trihydrate crystals melt at , and the liquid sodium acetate dissolves in the released water of crystallization
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is ...
. When heated past the melting point and subsequently allowed to cool, the aqueous solution becomes supersaturated
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a ...
. This solution is capable of cooling to room temperature without forming crystals. By pressing on a metal disc within the heating pad, a nucleation
In thermodynamics, nucleation is the first step in the formation of either a new Phase (matter), thermodynamic phase or Crystal structure, structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically def ...
center is formed, causing the solution to crystallize back into solid sodium acetate trihydrate. The process of crystallization is exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
. The latent heat of fusion
In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing energy, typically heat, to a specific quantity of the substance to change its state from a s ...
is about 264–289 kJ/kg.Ibrahim Dincer and Marc A. RosenThermal Energy Storage: Systems and Applications
page 155. Unlike some types of heat packs, such as those dependent upon irreversible chemical reactions, a sodium acetate heat pack can be easily reused by immersing the pack in boiling water for a few minutes, until the crystals are completely dissolved, and allowing the pack to slowly cool to room temperature.
Heat stores and batteries
Sodium acetate trihydrate can also be used as a phase-change material to store heat, especially to provide domestic hot water for heat pump applications. The heat store consists of a well-insulated container filled with the salt through which pass a pair of copper coils. One coil is used to melt the material by passing hot water from either solar thermal panels or aheat pump
A heat pump is a device that uses electricity to transfer heat from a colder place to a warmer place. Specifically, the heat pump transfers thermal energy using a heat pump and refrigeration cycle, cooling the cool space and warming the warm s ...
. Cold mains water passes through the other coil where its temperature is raised to 40 or 50 ˚C to provide water for washing or cleaning. This process can be cycled almost indefinitely.
Preparation
For laboratory use, sodium acetate is inexpensive and usually purchased instead of being synthesized. It is sometimes produced in a laboratory experiment by the reaction ofacetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, commonly in the 5–18% solution known as vinegar
Vinegar () is an aqueous solution of diluted acetic acid and trace compounds that may include flavorings. Vinegar typically contains from 5% to 18% acetic acid by volume. Usually, the acetic acid is produced by a double fermentation, converting ...
, with sodium carbonate
Sodium carbonate (also known as washing soda, soda ash, sal soda, and soda crystals) is the inorganic compound with the formula and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water ...
("washing soda"), sodium bicarbonate
Sodium bicarbonate ( IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda (or simply “bicarb” especially in the UK) is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cat ...
("baking soda"), or sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
("lye", or "caustic soda"). Any of these reactions produce sodium acetate and water or sodium acetate and carbonic acid. When a sodium and carbonate ion-containing compound is used as the reactant, the carbonate anion (from sodium bicarbonate or carbonate) reacts with the hydrogen from the carboxyl group (-COOH) in acetic acid, forming carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
. Carbonic acid readily decomposes under normal conditions into gaseous carbon dioxide and water. This is the reaction taking place in the well-known "volcano" that occurs when the household products, baking soda and vinegar, are combined.
:CH3COOH + NaHCO3 → CH3COONa +
: → +
Industrially, sodium acetate trihydrate is prepared by reacting acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
with sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
using water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
as the solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
.
:CH3COOH + NaOH → CH3COONa + H2O.
To manufacture anhydrous sodium acetate industrially, the Niacet Process is used. Sodium metal ingots are extruded through a die to form a ribbon of sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
metal, usually under an inert gas atmosphere such as N2, and then immersed in anhydrous acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
.
:2 CH3COOH + 2 Na →2 CH3COONa + H2.
The hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
gas is normally a valuable byproduct.
Structure
Thecrystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
of anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
sodium acetate has been described as alternating sodium-carboxylate and methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated a ...
layers. Sodium acetate trihydrate's structure consists of distorted octahedral coordination at sodium. Adjacent octahedra share edges to form one-dimensional chains. Hydrogen bonding
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
in two dimensions between acetate ions and water of hydration links the chains into a three-dimensional network.
Reactions
Sodium acetate can be used to form anester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
with an alkyl halide such as bromoethane:
: CH3COONa + BrCH2CH3 → CH3COOCH2CH3 + NaBr
Sodium acetate undergoes decarboxylation to form methane (CH4) under forcing conditions (pyrolysis in the presence of sodium hydroxide):
: CH3COONa + NaOH → CH4 + Na2CO3
Calcium oxide is the typical catalyst used for this reaction.
Caesium salts also catalyze this reaction.
References
External links
Hot Ice – Instructions, Pictures, and Videos
* {{Authority control Acetates E-number additives Food additives Organic sodium salts Photographic chemicals