|
Caesium Peroxide
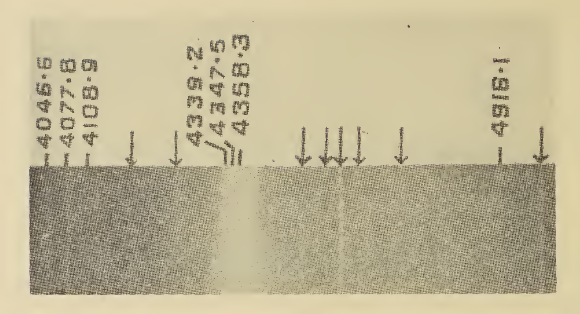
Caesium peroxide or cesium peroxide is a compound of caesium and oxygen. It can be formed from caesium metal by adding a stoichiometric amount in ammonia solution, or oxidizing the solid metal directly. :2Cs + O2 → Cs2O2 It can also be formed by the thermal decomposition of caesium superoxide: :2CsO2 → Cs2O2 + O2 Upon heating until 650°C, the compound will decompose to caesium monoxide and atomic oxygen: :\mathsf Caesium peroxide shows a Raman vibration at 743 cm−1, due to the presence of the peroxide ions. The compound is often used as a coating for photocathodes, due to its low work function In solid-state physics, the work function (sometimes spelt workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" m .... References Peroxides Caesium compounds {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Peroxide
Lithium peroxide is the inorganic compound with the formula Li2 O2. It is a white, nonhygroscopic solid. Because of its high oxygen:mass and oxygen:volume ratios, the solid has been used to remove CO2 from the atmosphere in spacecraft. Preparation It is prepared by the reaction of hydrogen peroxide and lithium hydroxide. This reaction initially produces lithium hydroperoxide:E. Dönges "Lithium and Sodium Peroxides" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 979. :LiOH + H2O2 → LiOOH + H2O This lithium hydroperoxide has also been described as lithium peroxide monoperoxohydrate trihydrate (Li2O2·H2O2·3H2O). Dehydration of this material gives the anhydrous peroxide salt: :2 LiOOH → Li2O2 + H2O2 Li2O2 decomposes at about 450 °C to give lithium oxide: :2 Li2O2 → 2 Li2O + O2 The structure of solid Li2O2 has been determined by X-ray crystallography and density functional theory. The solid features a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work Function
In solid-state physics, the work function (sometimes spelt workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" means that the final electron position is far from the surface on the atomic scale, but still too close to the solid to be influenced by ambient electric fields in the vacuum. The work function is not a characteristic of a bulk material, but rather a property of the surface of the material (depending on crystal face and contamination). Definition The work function for a given surface is defined by the difference :W = -e\phi - E_, where is the charge of an electron, is the electrostatic potential in the vacuum nearby the surface, and is the Fermi level (electrochemical potential of electrons) inside the material. The term is the energy of an electron at rest in the vacuum nearby the surface. In practice, one directly control ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photocathode
A photocathode is a surface engineered to convert light (photons) into electrons using the photoelectric effect. Photocathodes are important in accelerator physics where they are utilised in a photoinjector to generate high brightness electron beams. Electron beams generated with photocathodes are commonly used for free electron lasers and for ultrafast electron diffraction. Photocathodes are also commonly used as the negatively charged electrode in a light detection device such as a photomultiplier or phototube. Important Properties Quantum Efficiency (QE) Quantum efficiency is a unitless number that measures the sensitivity of the photocathode to light. It is the ratio of the number of electrons emitted to the number of incident photons.Rao, T., & Dowell, D. H. (2013). ''An engineering guide to photoinjectors''. CreateSpace Independent Publishing. This property depends on the wavelength of light being used to illuminate the photocathode. For many applications, QE is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable. The most common peroxide is hydrogen peroxide (), colloquially known simply as "peroxide". It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well. In addition to hydrogen peroxide, some other major classes of peroxides are: * Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate. * Main group peroxides, compounds with the linkage (E = main group element). * Metal peroxides, examples being barium peroxide (), sodium peroxide () and zinc peroxide (). * Organic peroxide In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raman Spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified. Raman spectroscopy relies upon inelastic scattering of photons, known as Raman scattering. A source of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range is used, although X-rays can also be used. The laser light interacts with molecular vibrations, phonons or other excitations in the system, resulting in the energy of the laser photons being shifted up or down. The shift in energy gives information about the vibrational modes in the system. Infrared spectroscopy typically yields similar yet complementary information. Typically, a sample is illuminated with a laser beam. Elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Oxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are: *Atomic oxygen (O1), a free radical. * Singlet oxygen (O2*), one of two metastable states of molecular oxygen. * Tetraoxygen (O4), another metastable form. *Solid oxygen, existing in six variously colored phases, of which one is and another one metallic. Atomic oxygen Atomic oxygen, denoted O(3P) or O(3P), is very reactive, as the single atoms of oxygen tend to quickly bond with nearby molecules. On Earth's surface, it exists naturally for a very short time. In outer space, the presence of ample ultraviolet radiation results in a low Earth orbit atmosphere in which 96% of the oxygen occurs in atomic form. Ryan D. McCulla, Saint Louis University (2010). /acswebcontent.acs.org/prfar/2010/reports/P11141.html "Atomic Oxygen O( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Monoxide
Caesium monoxide, (Cs2O), often simply Caesium oxide, is the simplest and most common oxide of the caesium. It forms yellow-orange hexagonal crystals. Uses Caesium oxide is used in photocathodes to detect infrared signals in devices such as image intensifiers, vacuum photodiodes, photomultipliers, and TV camera tubes L. R. Koller described the first modern photoemissive surface in 1929–30 as a layer of caesium on a layer of caesium oxide on a layer of silver. It is a good electron emitter; however, its high vapor pressure limits its usefulness. Reactions Elemental magnesium reduces caesium oxide to elemental caesium, forming magnesium oxide Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions ... as a side-product: :Cs2O + Mg → 2Cs + MgO Cs2O is hygroscopic, forming the cor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Superoxide
Caesium superoxide is the superoxide of caesium. It is an orange solid. Preparation Burning caesium in excess oxygen will produce caesium superoxide. : Properties Caesium superoxide's crystal structure is same as calcium carbide. It contains direct oxygen-oxygen bonding. It reacts with water to form hydrogen peroxide and caesium hydroxide. : The standard enthalpy of formation ΔHf0 of caesium superoxide is −295 kJ/mol. Caesium superoxide reacts with ozone Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lowe ... to form caesium ozonide. : References Caesium compounds Superoxides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Peroxide
Sodium peroxide is an inorganic compound with the formula Na2O2. This yellowish solid is the product of sodium ignited in excess oxygen. It is a strong base. This metal peroxide exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and Na2O2·8H2O.Harald Jakob, Stefan Leininger, Thomas Lehmann, Sylvia Jacobi, Sven Gutewort. "Peroxo Compounds, Inorganic". ''Ullmann's Encyclopedia of Industrial Chemistry'', 2007, Wiley-VCH, Weinheim. . The octahydrate, which is simple to prepare, is white, in contrast to the anhydrous material. Properties Sodium peroxide crystallizes with hexagonal symmetry. Upon heating, the hexagonal form undergoes a transition into a phase of unknown symmetry at 512 °C. With further heating above the 657 °C boiling point, the compound decomposes to Na2O, releasing O2.Lewis, R. J. ''Sax's Dangerous Properties of Industrial Materials, 10th ed.'', John Wiley & Sons, Inc.: 2000. : 2 Na2O2 → 2 Na2O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



-3D-balls.png)