|
TNNT3
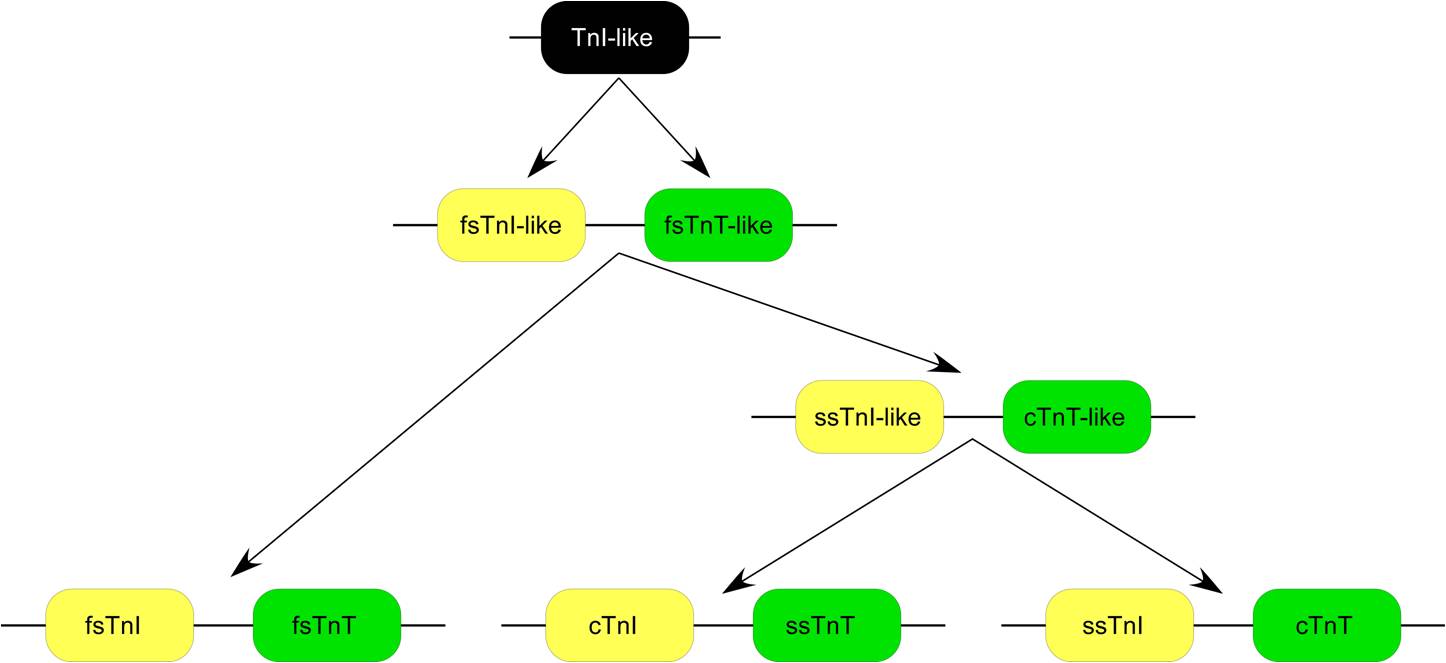
Fast skeletal muscle troponin T (fTnT) is a protein that in humans is encoded by the ''TNNT3'' gene. The TNNT3 gene is located at 11p15.5 in the human genome, encoding the fast skeletal muscle isoform of troponin T (fsTnT). fsTnT is an ~31-kDa protein consisting of 268 amino acids including the first methionine with an isoelectric point (pI) of 6.21 (embryonic form). fsTnT is the tropomyosin-binding and thin filament anchoring subunit of the troponin complex in the sarcomeres of fast twitch skeletal muscle. TNNT3 gene is specifically expressed in vertebrate fast twitch skeletal muscles. Evolution TNNT3 gene evolved as one of the three TnT isoform genes in vertebrates. Each of the TnT isoform genes is linked to an upstream troponin I (TnI, one of the other two subunits of the troponin complex) isoform gene, and fsTnT is linked with fsTnI genes (Fig. 1). Sequence homology and protein epitope allosteric similarity data suggest that TnT gene was originated by duplication of a TnI- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TnT TnI Gene Pairs
Troponin T (shortened TnT or TropT) is a part of the troponin complex, which are proteins integral to the contraction of skeletal and heart muscles. They are expressed in skeletal and cardiac myocytes. Troponin T binds to tropomyosin and helps position it on actin, and together with the rest of the troponin complex, modulates contraction of striated muscle. The cardiac subtype of troponin T is especially useful in the laboratory diagnosis of heart attack because it is released into the blood-stream when damage to heart muscle occurs. It was discovered by the German physician Hugo A. Katus at the University of Heidelberg, who also developed the troponin T assay. Subtypes * Slow skeletal troponin T1, TNNT1 (19q13.4, ) * Cardiac troponin T2, TNNT2 (1q32, ) * Fast skeletal troponin T3, TNNT3 (11p15.5, ) Reference values The 99th percentile cutoff for cardiac troponin T (cTnT) is 0.01 ng/mL. The reference range for the high sensitivity troponin T is a normal 52 ng/L. Background ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have unique functions. A set of protein isoforms may be formed from alternative splicings, variable promoter usage, or other post-transcriptional modifications of a single gene; post-translational modifications are generally not considered. (For that, see Proteoforms.) Through RNA splicing mechanisms, mRNA has the ability to select different protein-coding segments (exons) of a gene, or even different parts of exons from RNA to form different mRNA sequences. Each unique sequence produces a specific form of a protein. The discovery of isoforms could explain the discrepancy between the small number of protein coding regions of genes revealed by the human genome project and the large diversity of proteins seen in an organism: different proteins enc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
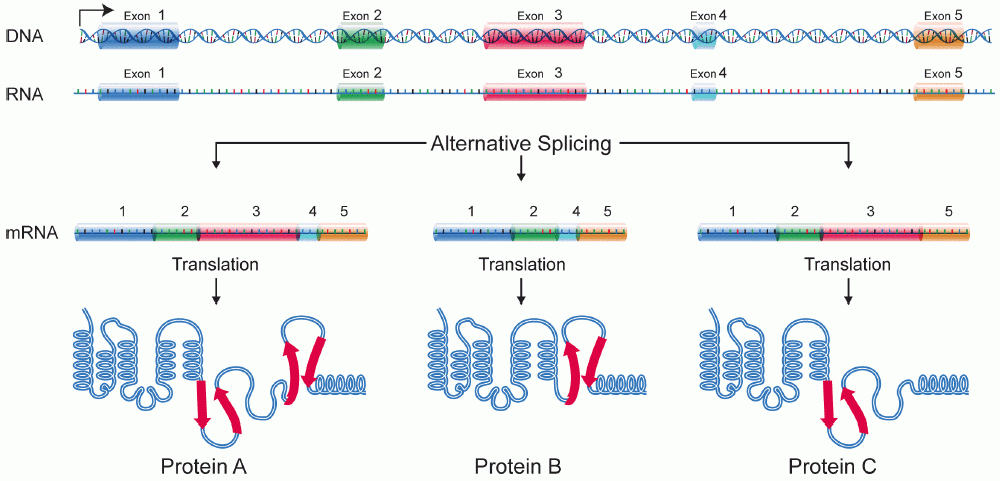
Alternative Splicing
Alternative splicing, alternative RNA splicing, or differential splicing, is an alternative RNA splicing, splicing process during gene expression that allows a single gene to produce different splice variants. For example, some exons of a gene may be included within or excluded from the final RNA product of the gene. This means the exons are joined in different combinations, leading to different splice variants. In the case of protein-coding genes, the proteins translated from these splice variants may contain differences in their amino acid sequence and in their biological functions (see Figure). Biologically relevant alternative splicing occurs as a normal phenomenon in eukaryotes, where it increases the number of proteins that can be encoded by the genome. In humans, it is widely believed that ~95% of multi-exonic genes are alternatively spliced to produce functional alternative products from the same gene but many scientists believe that most of the observed splice variants ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TNNT2
Cardiac muscle troponin T (cTnT) is a protein that in humans is encoded by the ''TNNT2'' gene. Cardiac TnT is the tropomyosin-binding subunit of the troponin complex, which is located on the thin filament of striated muscles and regulates muscle contraction in response to alterations in intracellular calcium ion concentration. The TNNT2 gene is located at 1q32 in the human chromosomal genome, encoding the cardiac muscle isoform of troponin T (cTnT). Human cTnT is an ~36-kDa protein consisting of 297 amino acids including the first methionine with an isoelectric point (pI) of 4.88. It is the tropomyosin- binding and thin filament anchoring subunit of the troponin complex in cardiac muscle cells. TNNT2 gene is expressed in vertebrate cardiac muscles and embryonic skeletal muscles. Structure Cardiac TnT is a 35.9 kDa protein composed of 298 amino acids. Cardiac TnT is the largest of the three troponin subunits (cTnT, TNNI3, troponin I (TnI), TNNC1, troponin C (TnC)) on the ACTC1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TNNT1
Slow skeletal muscle troponin T (sTnT) is a protein that in humans is encoded by the ''TNNT1'' gene. The TNNT1 gene is located at 19q13.4 in the human chromosomal genome, encoding the slow twitch skeletal muscle isoform of troponin T (ssTnT). ssTnT is an ~32-kDa protein consisting of 262 amino acids (including the first methionine) with an isoelectric point (pI) of 5.95. It is the tropomyosin binding and thin filament anchoring subunit of the troponin complex in the sarcomeres of slow twitch skeletal muscle fibers. TNNT1 gene is specifically expressed in slow skeletal muscle of vertebrates, with one exception that dry land toad (Bufo) cardiac muscle expresses ssTnT other than cardiac TnT. Evolution Three homologous genes have evolved in vertebrates, encoding three muscle type specific isoforms A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perfo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Troponin I
Troponin I is a cardiac and skeletal muscle protein family. It is a part of the troponin protein complex, where it binds to actin in thin myofilaments to hold the actin-tropomyosin complex in place. Troponin I prevents myosin from binding to actin in relaxed muscle. When calcium binds to the troponin C, it causes conformational changes which lead to dislocation of troponin I. Afterwards, tropomyosin leaves the binding site for myosin on actin leading to contraction of muscle. The letter ''I'' is given due to its inhibitory character. It is a useful marker in the laboratory diagnosis of heart attack. It occurs in different plasma concentration but the same circumstances as troponin T - either test can be performed for confirmation of cardiac muscle damage and laboratories usually offer one test or the other. Three paralogs with unique tissue-specific expression patterns are expressed in humans, listed below with their locations and OMIM accessions: * Slow-twitch skeletal muscle i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and non-coding genes. During gene expression (the synthesis of Gene product, RNA or protein from a gene), DNA is first transcription (biology), copied into RNA. RNA can be non-coding RNA, directly functional or be the intermediate protein biosynthesis, template for the synthesis of a protein. The transmission of genes to an organism's offspring, is the basis of the inheritance of phenotypic traits from one generation to the next. These genes make up different DNA sequences, together called a genotype, that is specific to every given individual, within the gene pool of the population (biology), population of a given species. The genotype, along with environmental and developmental factors, ultimately determines the phenotype ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
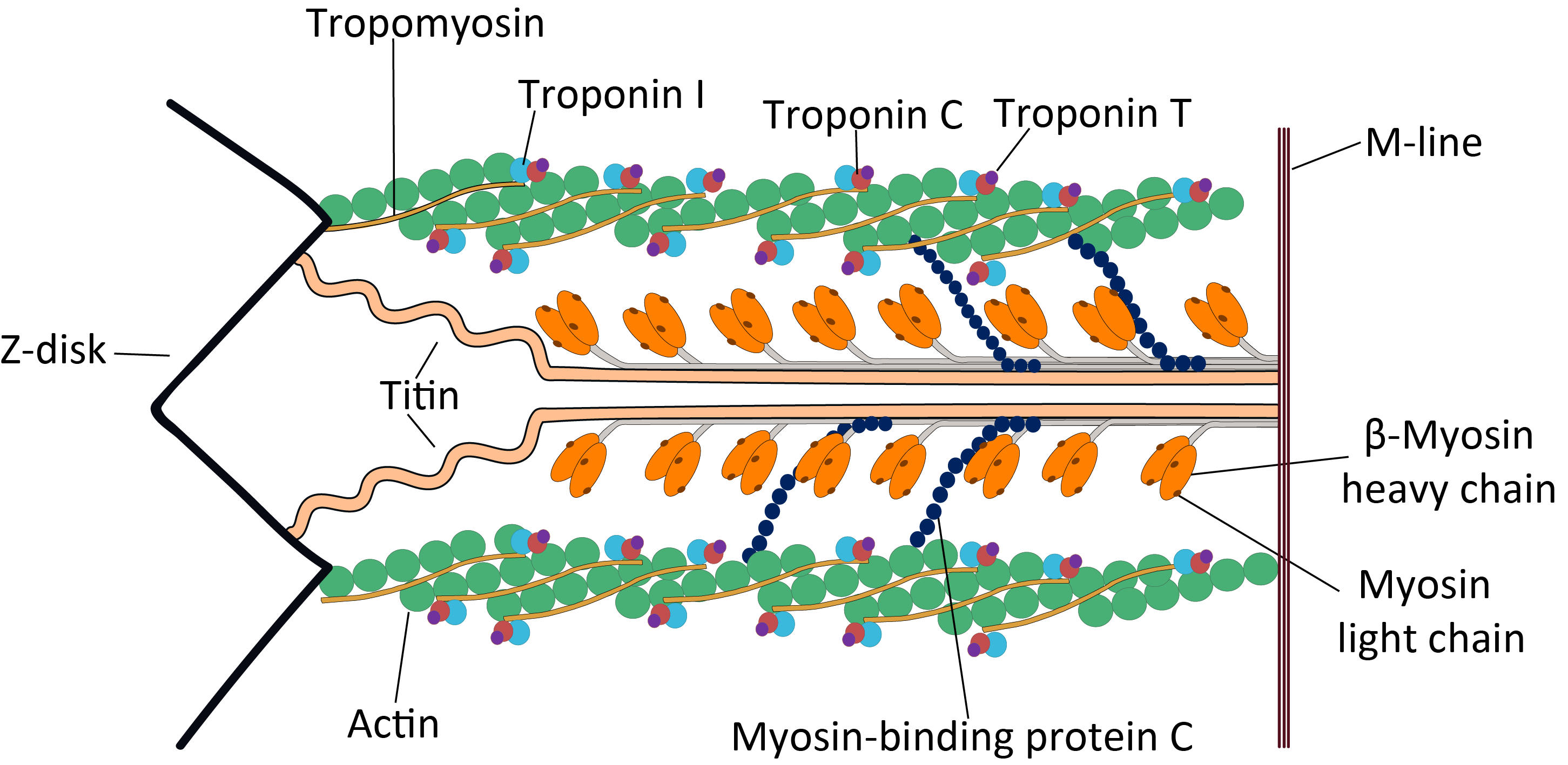
Sarcomere
A sarcomere (Greek σάρξ ''sarx'' "flesh", μέρος ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal striated muscle, Skeletal muscles are composed of tubular muscle cells (called muscle fibers or myofibers) which are formed during embryonic development, embryonic myogenesis. Muscle fibers contain numerous tubular myofibrils. Myofibrils are composed of repeating sections of sarcomeres, which appear under the microscope as alternating dark and light bands. Sarcomeres are composed of long, fibrous proteins as filaments that slide past each other when a muscle contracts or relaxes. The costamere is a different component that connects the sarcomere to the sarcolemma. Two of the important proteins are myosin, which forms the thick filament, and actin, which forms the thin filament. Myosin has a long fibrous tail and a globular head that binds to actin. The myosin head also binds to Adenosine triphos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Troponin Complex
Troponin, or the troponin complex, is a complex of three regulatory proteins (troponin C, troponin I, and troponin T) that are integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle. Measurements of cardiac-specific troponins I and T are extensively used as diagnostic and prognostic indicators in the management of myocarditis, myocardial infarction and acute coronary syndrome. Blood troponin levels may be used as a diagnostic marker for stroke or other myocardial injury that is ongoing, although the sensitivity of this measurement is low. Function Troponin is attached to the protein tropomyosin and lies within the groove between actin filaments in muscle tissue. In a relaxed muscle, tropomyosin blocks the attachment site for the myosin crossbridge, thus preventing contraction. When the muscle cell is stimulated to contract by an action potential, calcium channels open in the sarcoplasmic membrane and release calcium into the sarcoplasm. Some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |