|
Saltwater Soap
Saltwater soap, also called sailors' soap, is a potassium-based soap for use with seawater. Inexpensive common commercial soap will not lather or dissolve in seawater due to high levels of sodium chloride in the water. Similarly, common soap does not work as well as potassium-based soap in hard water where calcium replaces the sodium, making residual insoluble "scum" due to the insolubility of the soap residue. To be an effective cleaning agent, soap must be able to dissolve in water. Ordinary soap is a salt of a fatty acid. Soaps are mainly used as surfactants for washing, bathing, and cleaning. Soaps for cleansing are made by treating vegetable or animal oils and fats with a strongly alkaline solution. Fats and oils are composed of triglycerides; three molecules of fatty acids are attached to a single molecule of glycerol.Cavitch, Susan Miller. ''The Natural Soap Book''. Storey Publishing, 1994 . The alkaline solution, which is often called lye (although the term "lye soap" r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthocl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Murphy Oil Soap
Murphy Oil Soap is an American brand of cleaning product that is manufactured by Colgate-Palmolive. In 1910, Jeremiah Murphy, director of the Phoenix Oil Company, bought the formula for Murphy Oil Soap from a recent immigrant from Germany. The soap, with its potassium vegetable oil base, and no phosphates, proved to be very popular in Ohio. The company continued to be run by the Murphy family for 80 years, when they sold to Colgate. It is available in a concentrated liquid form which is then mixed with water, as well as pre-diluted form which comes in a trigger spray bottle. Commercials for the product state that the product is ideal for cleaning wood surfaces. The other constituents of Murphy Oil Soap are sodium EDTA, propylene glycol, fragrance, surfactants, and water.THE MURPHY-PHOENIX -- MURPHY OIL SOAP LIQUID MATERIAL SAFETY DATA SHEET as published bOHIO STATE UNIVERSITY COLLEGE OF BIOLOGICAL SCIENCES. FSC: 9160. NIIN: 00B130042. Manufacturer's CAGE: 5R288. MSDS Serial Numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Soap
Lithium soap is a soap consisting of a lithium salt of a fatty acid. Sodium-based and potassium-based soaps are used as cleaning agents in domestic and industrial applications, whereas lithium soaps are used as components of lithium grease (white lithium). Lithium soaps are produced by saponification of triglycerides, using lithium hydroxide or lithium carbonate as the saponification agent. Lithium soaps are used as lubricant components and form-release agents at relatively high temperatures. The main components of lithium soaps are lithium stearate and lithium 12-hydroxystearate. Lithium grease Lubricating greases are commonly formulated as mixtures of an oil and a lithium soap thickener.Angelo Nora, Alfred Szczepanek, Gunther Koenen, "Metallic Soaps" in Ullmann’s Encyclopedia of Industrial Chemistry 2005 Wiley-VCH, Weinheim. Some formulations include PTFE or other substances, such as molybdenum disulfide. Lithium grease adheres particularly well to metal, is non-co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Feldspar
Feldspars are a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) feldspars and the ''alkali'' (potassium-sodium) feldspars. Feldspars make up about 60% of the Earth's crust, and 41% of the Earth's continental crust by weight. Feldspars crystalize from magma as both intrusive and extrusive igneous rocks and are also present in many types of metamorphic rock. Rock formed almost entirely of calcic plagioclase feldspar is known as anorthosite. Feldspars are also found in many types of sedimentary rocks. Compositions The feldspar group of minerals consists of tectosilicates, silicate minerals in which silicon ions are linked by shared oxygen ions to form a three-dimensional network. Compositions of major elements in common feldspars can be expressed in terms of three endmembers: * potassium feldspar (K- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporator (marine)
An evaporator, distiller or distilling apparatus is a piece of ship's equipment used to produce fresh drinking water from sea water by distillation. As fresh water is bulky, may spoil in storage, and is an essential supply for any long voyage, the ability to produce more fresh water in mid-ocean is important for any ship. Early evaporators on sailing vessels Although distillers are often associated with steam ships, their use pre-dates this. Obtaining fresh water from seawater is a theoretically simple system that, in practice, presented many difficulties. While there are numerous effective methods today, early desalination efforts had low yields and often could not produce potable water. At first, only larger warships and some exploratory ships were fitted with distilling apparatus: a warship's large crew naturally needed a large supply of water, more than they could stow on board in advance. Cargo ships, with their smaller crews, merely carried their supplies with them. A se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elephant Toothpaste
Elephant's toothpaste is a foamy substance caused by the rapid decomposition of hydrogen peroxide () using potassium iodide (KI) or yeast and warm water as a catalyst. How rapidly the reaction proceeds will depend on the concentration of hydrogen peroxide. Because it requires only a small number of ingredients and makes a "volcano of foam", this is a popular experiment for children to perform in school or at parties. The experiment is also known as the "marshmallow experiment", but is unrelated to the psychological Stanford marshmallow experiment. Explanation Description About 50 ml of concentrated (>12%) hydrogen peroxide is first mixed with liquid soap or dishwashing detergent. Then, a catalyst, often around 10 ml potassium iodide solution or catalase from baker's yeast, is added to make the hydrogen peroxide decompose very quickly. Hydrogen peroxide breaks down into oxygen and water. As a small amount of hydrogen peroxide generates a large volume of oxygen, the oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Conservation
Water conservation includes all the policies, strategies and activities to sustainably manage the natural resource of fresh water, to protect the hydrosphere, and to meet the current and future human demand (thus avoiding water scarcity). Population, household size and growth and affluence all affect how much water is used. Factors such as climate change have increased pressures on natural water resources especially in manufacturing and agricultural irrigation. Many countries have already implemented policies aimed at water conservation, with much success. The key activities to conserve water are as follows: any beneficial reduction in water loss, use and waste of resources, avoiding any damage to water quality; and improving water management practices that reduce the use or enhance the beneficial use of water. Technology solutions exist for households, commercial and agricultural applications. Water conservation programs involved in social solutions are typically initiated at t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
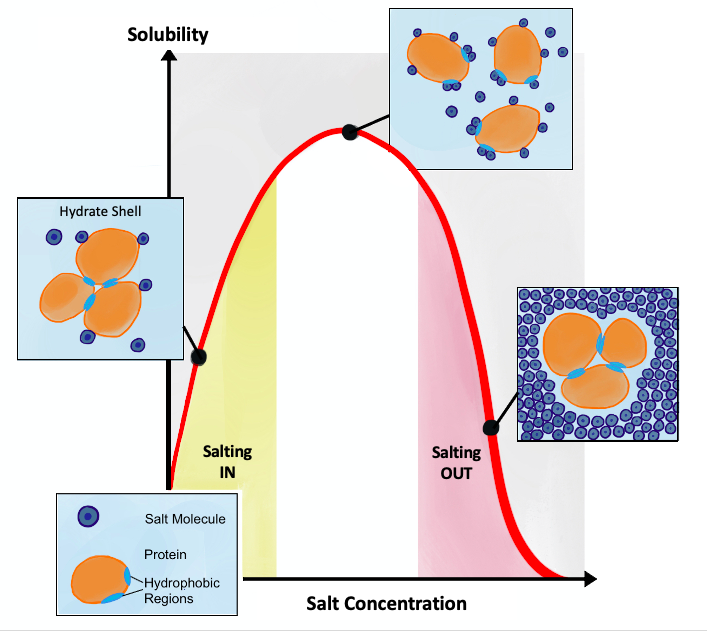
Salting Out
Salting out (also known as salt-induced precipitation, salt fractionation, anti-solvent crystallization, precipitation crystallization, or drowning out) is a purification technique that utilizes the reduced solubility of certain molecules in a solution of very high ionic strength. Salting out is typically used to precipitate large biomolecules, such as proteins or DNA. Because the salt concentration needed for a given protein to precipitate out of the solution differs from protein to protein, a specific salt concentration can be used to precipitate a target protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed. Principle Salt compounds dissociate in aqueous solutions. This property is exploited in the process of salting out. When the salt concentration is increased, some of the water molecules are attracted by the salt ions, which decreases the number of water molecules available to interact with the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Common Ion Effect
The common-ion effect refers to the decrease in solubility of an ionic precipitate by the addition to the solution of a soluble compound with an ion in common with the precipitate. This behaviour is a consequence of Le Chatelier's principle for the equilibrium reaction of the ionic association/dissociation. The effect is commonly seen as an effect on the solubility of salts and other weak electrolytes. Adding an additional amount of one of the ions of the salt generally leads to increased precipitation of the salt, which reduces the concentration of both ions of the salt until the solubility equilibrium is reached. The effect is based on the fact that both the original salt and the other added chemical have one ion in common with each other. Examples of the common-ion effect Dissociation of hydrogen sulfide in presence of hydrochloric acid Hydrogen sulfide (H2S) is a weak electrolyte. It is partially ionized when in aqueous solution, therefore there exists an equilibrium betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and read ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |