Hydrolysis on:
[Wikipedia]
[Google]
[Amazon]
 Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a
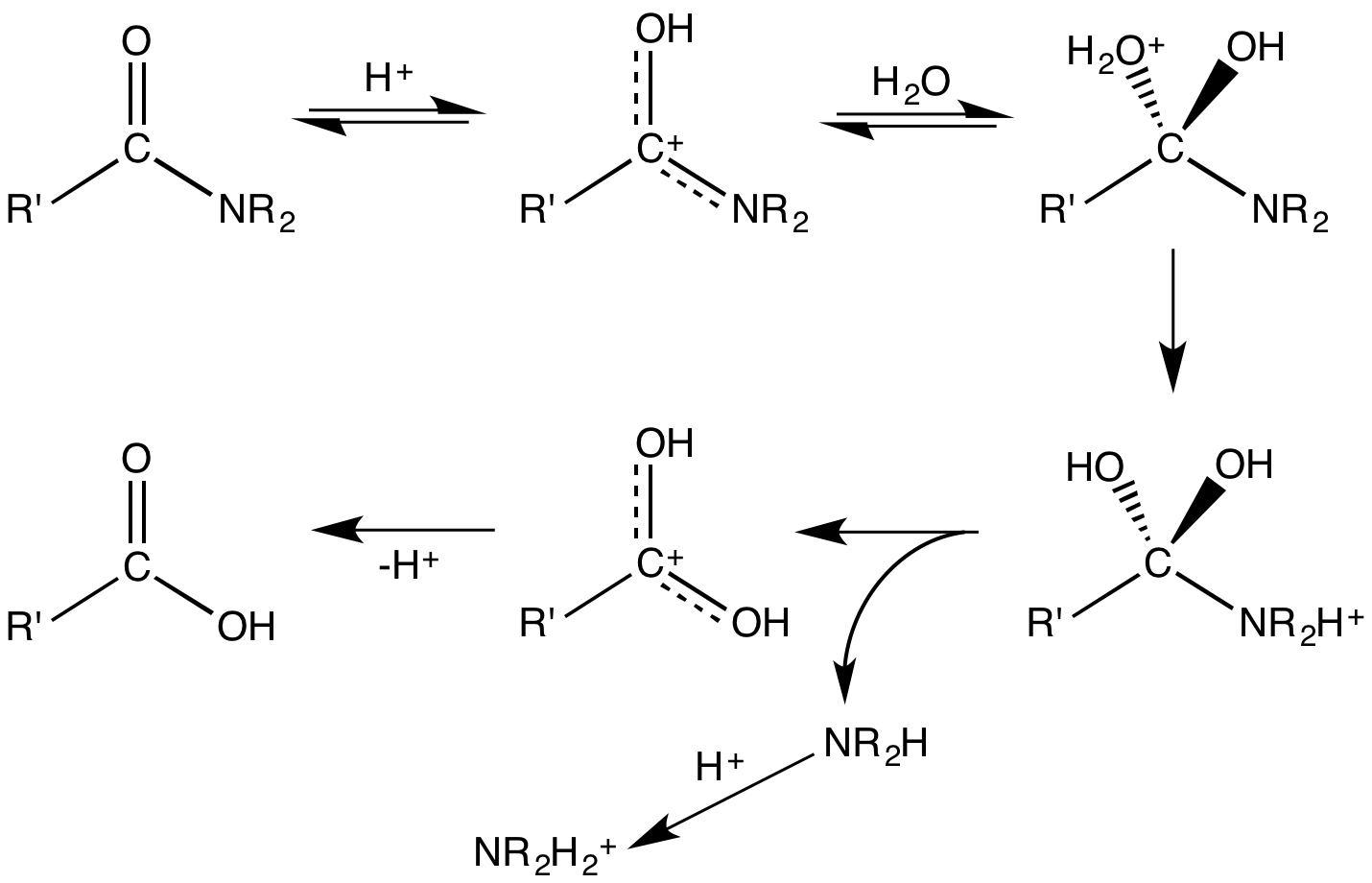 Upon hydrolysis, an
Upon hydrolysis, an
ATP + H2O -> ADP + P_
Secondly, the removal of a terminal diphosphate to yield
 Monosaccharides can be linked together by
Monosaccharides can be linked together by
M(H2O)_\mathit^ + H2O <=> M(H2O)_(OH)^ + H3O+
Thus the aqua cations behave as acids in terms of Brønsted–Lowry acid–base theory. This effect is easily explained by considering the
 Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or m ...
is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
and fructose), this is recognized as saccharification
In chemistry, saccharification is a term for denoting any chemical change wherein a monosaccharide molecule remains intact after becoming unbound from another saccharide.
For example, when a carbohydrate is broken into its component sugar molecul ...
.
Hydrolysis reactions can be the reverse of a condensation reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a ...
in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water.
Types
Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. In such reactions, one fragment of the target molecule (or parent molecule) gains a hydrogen ion. It breaks a chemical bond in the compound.Salts
A common kind of hydrolysis occurs when asalt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
of a weak acid or weak base
A weak base is a base that, upon dissolution in water, does not dissociate completely, so that the resulting aqueous solution contains only a small proportion of hydroxide ions and the concerned basic radical, and a large proportion of undissociat ...
(or both) is dissolved in water. Water spontaneously ionizes into hydroxide anions and hydronium cations. The salt also dissociates into its constituent anions and cations. For example, sodium acetate
Sodium acetate, CH3COONa, also abbreviated Na O Ac, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Applications
Biotechnological
Sodium acetate is used as the carbon source for culturing bacteria ...
dissociates in water into sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
and acetate ions. Sodium ions react very little with the hydroxide ions whereas the acetate ions combine with hydronium ions to produce acetic acid. In this case the net result is a relative excess of hydroxide ions, yielding a basic solution.
Strong acids
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions ...
also undergo hydrolysis. For example, dissolving sulfuric acid () in water is accompanied by hydrolysis to give hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid i ...
and bisulfate, the sulfuric acid's conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
. For a more technical discussion of what occurs during such a hydrolysis, see Brønsted–Lowry acid–base theory.
Esters and amides
Acid–base-catalysed hydrolyses are very common; one example is the hydrolysis ofamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ...
s or ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
s. Their hydrolysis occurs when the nucleophile (a nucleus-seeking agent, e.g., water or hydroxyl ion) attacks the carbon of the carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
of the ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
or amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ...
. In an aqueous base, hydroxyl ions are better nucleophiles than polar molecules such as water. In acids, the carbonyl group becomes protonated, and this leads to a much easier nucleophilic attack. The products for both hydrolyses are compounds with carboxylic acid groups.
Perhaps the oldest commercially practiced example of ester hydrolysis is saponification
Saponification is a process of converting esters into soaps and alcohols by the action of aqueous alkali (for example, aqueous sodium hydroxide solutions). Soaps are salts of fatty acids, which in turn are carboxylic acids with long carbon chains. ...
(formation of soap). It is the hydrolysis of a triglyceride
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from ''wikt:tri-#Prefix, tri-'' and ''glyceride'').
Triglycerides are the main constituents of body fat in humans and other ...
(fat) with an aqueous base such as sodium hydroxide (NaOH). During the process, glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
is formed, and the fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
s react with the base, converting them to salts. These salts are called soaps, commonly used in households.
In addition, in living systems, most biochemical reactions (including ATP hydrolysis) take place during the catalysis of enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s. The catalytic action of enzymes allows the hydrolysis of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s, fats, oils, and carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or m ...
s. As an example, one may consider protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the ...
s (enzymes that aid digestion
Digestion is the breakdown of large insoluble food molecules into small water-soluble food molecules so that they can be absorbed into the watery blood plasma. In certain organisms, these smaller substances are absorbed through the small intest ...
by causing hydrolysis of peptide bonds in protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s). They catalyze the hydrolysis of interior peptide bonds in peptide chains, as opposed to exopeptidase
An exopeptidase is any peptidase that catalyzes the cleavage of the terminal (or the penultimate) peptide bond; the process releases a single amino acid, dipeptide or a tripeptide from the peptide chain. Depending on whether the amino acid is rel ...
s (another class of enzymes, that catalyze the hydrolysis of terminal peptide bonds, liberating one free amino acid at a time).
However, proteases do not catalyze the hydrolysis of all kinds of proteins. Their action is stereo-selective: Only proteins with a certain tertiary structure are targeted as some kind of orienting force is needed to place the amide group in the proper position for catalysis. The necessary contacts between an enzyme and its substrates (proteins) are created because the enzyme folds in such a way as to form a crevice into which the substrate fits; the crevice also contains the catalytic groups. Therefore, proteins that do not fit into the crevice will not undergo hydrolysis. This specificity preserves the integrity of other proteins such as hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are require ...
s, and therefore the biological system continues to function normally.
 Upon hydrolysis, an
Upon hydrolysis, an amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ...
converts into a carboxylic acid and an amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
or ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
(which in the presence of acid are immediately converted to ammonium salts). One of the two oxygen groups on the carboxylic acid are derived from a water molecule and the amine (or ammonia) gains the hydrogen ion. The hydrolysis of peptides gives amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
s.
Many polyamide polymers such as nylon 6,6 hydrolyze in the presence of strong acids. The process leads to depolymerization Depolymerization (or depolymerisation) is the process of converting a polymer into a monomer or a mixture of monomers. This process is driven by an increase in entropy.
Ceiling temperature
The tendency of polymers to depolymerize is indicated by ...
. For this reason nylon products fail by fracturing when exposed to small amounts of acidic water. Polyesters are also susceptible to similar polymer degradation
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycl ...
reactions. The problem is known as environmental stress cracking.
ATP
Hydrolysis is related toenergy metabolism
Bioenergetics is a field in biochemistry and cell biology that concerns energy flow through living systems. This is an active area of biological research that includes the study of the transformation of energy in living organisms and the study of ...
and storage. All living cells require a continual supply of energy for two main purposes: the biosynthesis of micro and macromolecules, and the active transport of ions and molecules across cell membranes. The energy derived from the oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of nutrients is not used directly but, by means of a complex and long sequence of reactions, it is channeled into a special energy-storage molecule, adenosine triphosphate (ATP). The ATP molecule contains pyrophosphate
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P–O–P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate (Na2H2P2O7) and tetrasodium pyrophosphate (Na4P2O7), among othe ...
linkages (bonds formed when two phosphate units are combined) that release energy when needed. ATP can undergo hydrolysis in two ways: Firstly, the removal of terminal phosphate to form adenosine diphosphate (ADP) and inorganic phosphate, with the reaction:
: adenosine monophosphate
Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine; it is an ester of phosphoric acid and the nucleoside adenosine. As a substit ...
(AMP) and pyrophosphate
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P–O–P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate (Na2H2P2O7) and tetrasodium pyrophosphate (Na4P2O7), among othe ...
. The latter usually undergoes further cleavage into its two constituent phosphates. This results in biosynthesis reactions, which usually occur in chains, that can be driven in the direction of synthesis when the phosphate bonds have undergone hydrolysis.
Polysaccharides
glycosidic bond
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
A glycosidic bond is formed between the hemiacetal or hemiketal group ...
s, which can be cleaved by hydrolysis. Two, three, several or many monosaccharides thus linked form disaccharide
A disaccharide (also called a double sugar or ''biose'') is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, la ...
s, trisaccharides, oligosaccharides, or polysaccharides, respectively. Enzymes that hydrolyze glycosidic bonds are called " glycoside hydrolases" or "glycosidases".
The best-known disaccharide is sucrose (table sugar). Hydrolysis of sucrose yields glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
and fructose. Invertase
Invertase is an enzyme that catalyzes the hydrolysis (breakdown) of sucrose (table sugar) into fructose and glucose. Alternative names for invertase include , saccharase, glucosucrase, beta-h-fructosidase, beta-fructosidase, invertin, sucrase, m ...
is a sucrase used industrially for the hydrolysis of sucrose to so-called invert sugar. Lactase
Lactase is an enzyme produced by many organisms. It is located in the brush border of the small intestine of humans and other mammals. Lactase is essential to the complete digestion of whole milk; it breaks down lactose, a sugar which gives ...
is essential for digestive hydrolysis of lactose in milk; many adult humans do not produce lactase and cannot digest the lactose in milk.
The hydrolysis of polysaccharides to soluble sugars can be recognized as saccharification
In chemistry, saccharification is a term for denoting any chemical change wherein a monosaccharide molecule remains intact after becoming unbound from another saccharide.
For example, when a carbohydrate is broken into its component sugar molecul ...
. Malt made from barley
Barley (''Hordeum vulgare''), a member of the grass family, is a major cereal grain grown in temperate climates globally. It was one of the first cultivated grains, particularly in Eurasia as early as 10,000 years ago. Globally 70% of barley p ...
is used as a source of β-amylase to break down starch into the disaccharide maltose, which can be used by yeast to produce beer. Other amylase
An amylase () is an enzyme that catalyses the hydrolysis of starch (Latin ') into sugars. Amylase is present in the saliva of humans and some other mammals, where it begins the chemical process of digestion. Foods that contain large amounts of ...
enzymes may convert starch to glucose or to oligosaccharides. Cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell w ...
is first hydrolyzed to cellobiose by cellulase and then cellobiose is further hydrolyzed to glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
by beta-glucosidase
β-Glucosidase (EC 3.2.1.21; systematic name β-D-glucoside glucohydrolase) is an enzyme that catalyses the following reaction:
: Hydrolysis of terminal, non-reducing β-D-glucosyl residues with release of β-D-glucose
Structure
β-Glucosidase ...
. Ruminants such as cows are able to hydrolyze cellulose into cellobiose and then glucose because of symbiotic bacteria that produce cellulases.
Metal aqua ions
Metal ions are Lewis acids, and in aqueous solution they formmetal aquo complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorat ...
es of the general formula . The aqua ions undergo hydrolysis, to a greater or lesser extent. The first hydrolysis step is given generically as
:inductive effect
In chemistry, the inductive effect in a molecule is a local change in the electron density due to electron-withdrawing or electron-donating groups elsewhere in the molecule, resulting in a permanent dipole in a bond.
It is present in a σ (sigma ...
of the positively charged metal ion, which weakens the bond of an attached water molecule, making the liberation of a proton relatively easy.
The dissociation constant, pKa, for this reaction is more or less linearly related to the charge-to-size ratio of the metal ion. Ions with low charges, such as are very weak acids with almost imperceptible hydrolysis. Large divalent ions such as , , and have a pKa of 6 or more and would not normally be classed as acids, but small divalent ions such as undergo extensive hydrolysis. Trivalent ions like and are weak acids whose pKa is comparable to that of acetic acid. Solutions of salts such as or in water are noticeably acidic
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a ...
; the hydrolysis can be suppressed by adding an acid such as nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
, making the solution more acidic.
Hydrolysis may proceed beyond the first step, often with the formation of polynuclear species via the process of olation. Some "exotic" species such as are well characterized. Hydrolysis tends to proceed as pH rises leading, in many cases, to the precipitation of a hydroxide such as or . These substances, major constituents of bauxite, are known as laterites and are formed by leaching from rocks of most of the ions other than aluminium and iron and subsequent hydrolysis of the remaining aluminium and iron.
Mechanism strategies
Acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...
s, imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
s, and enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
:
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and t ...
s can be converted back into ketones by treatment with excess water under acid-catalyzed conditions: ; ; .
See also
*Acid hydrolysis
In organic chemistry, acid hydrolysis is a hydrolysis process in which a protic acid is used to catalyze the cleavage of a chemical bond via a nucleophilic substitution reaction, with the addition of the elements of water (H2O). For example, in th ...
* Adenosine triphosphate
* Alkaline hydrolysis (body disposal)
* Catabolism
* Condensation reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a ...
* Dehydration reaction
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction.
Dehydration reactions in organic ch ...
* Hydrolysis constant
* Inhibitor protein
* Polymer degradation
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycl ...
* Proteolysis
* Saponification
Saponification is a process of converting esters into soaps and alcohols by the action of aqueous alkali (for example, aqueous sodium hydroxide solutions). Soaps are salts of fatty acids, which in turn are carboxylic acids with long carbon chains. ...
* Sol–gel polymerisation
* Solvolysis
In chemistry, solvolysis is a type of nucleophilic substitution (S1/S2) or elimination reaction, elimination where the nucleophile is a solvent molecule. Characteristic of S1 reactions, solvolysis of a chirality (chemistry), chiral reactant affor ...
* Thermal hydrolysis
Thermal hydrolysis is a process used for treating industrial waste, municipal solid waste and sewage sludge.
Description
Thermal hydrolysis is a two-stage process combining high-pressure boiling of waste or sludge followed by a rapid decompressio ...
References
{{Authority control Chemical reactions Equilibrium chemistry