|
SNCR
Selective non-catalytic reduction (SNCR) is a method to lessen nitrogen oxide emissions in conventional power plants that burn biomass, waste and coal. The process involves injecting either ammonia or urea into the firebox of the boiler at a location where the flue gas is between to react with the nitrogen oxides formed in the combustion process. The resulting product of the chemical redox reaction is molecular nitrogen (N2), carbon dioxide (CO2), and water (H2O). The conversion of noxious NOx to innocuous N2 is described by the following simplified equation: :4 NO + 4 NH3 + O2 → 4 N2 + 6 H2O When urea is used, the pre-reaction occurs to first convert it to ammonia: :NH2CONH2 + H2O → 2 NH3 + CO2 Being a solid, urea is easier to handle and store than the more dangerous ammonia (NH3), so it is the reactant of choice. The reaction requires a sufficient reaction time within a certain temperature range, typically , to be effective. At lower temperatures the NO and the ammonia do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
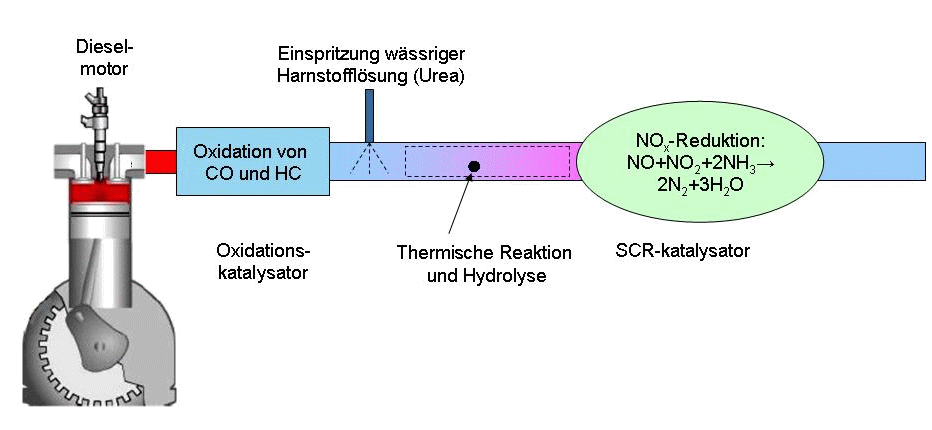
Selective Catalytic Reduction
Selective catalytic reduction (SCR) means converting nitrogen oxides, also referred to as with the aid of a catalyst into diatomic nitrogen (), and water (). A reductant, typically anhydrous ammonia (), aqueous ammonia (), or a urea () solution, is added to a stream of flue or exhaust gas and is reacted onto a catalyst. As the reaction drives toward completion, nitrogen (), and carbon dioxide (), in the case of urea use, are produced. Selective catalytic reduction of using ammonia as the reducing agent was patented in the United States by the Engelhard Corporation in 1957. Development of SCR technology continued in Japan and the US in the early 1960s with research focusing on less expensive and more durable catalyst agents. The first large-scale SCR was installed by the IHI Corporation in 1978.Steam: Its Generation and Uses. Babcock & Wilcox. Commercial selective catalytic reduction systems are typically found on large utility boilers, industrial boilers, and municipal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incineration
Incineration is a waste treatment process that involves the combustion of substances contained in waste materials. Industrial plants for waste incineration are commonly referred to as waste-to-energy facilities. Incineration and other high-temperature waste treatment systems are described as "thermal treatment". Incineration of waste materials converts the waste into ash, flue gas and heat. The ash is mostly formed by the inorganic constituents of the waste and may take the form of solid lumps or particulates carried by the flue gas. The flue gases must be cleaned of gaseous and particulate pollutants before they are dispersed into the atmosphere. In some cases, the heat that is generated by incineration can be used to generate electric power. Incineration with energy recovery is one of several waste-to-energy technologies such as gasification, pyrolysis and anaerobic digestion. While incineration and gasification technologies are similar in principle, the energy produce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the cellular metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. ''Urea'' is Neo-Latin, , , itself from Proto-Indo-European ''*h₂worsom''. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor base (chemistry), alkaline. The body uses it in many processes, most notably metabolic waste#Nitrogen wastes, nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Babcock & Wilcox
Babcock & Wilcox Enterprises, Inc. is an American energy technology and service provider that is active and has operations in many international markets with its headquarters in Akron, Ohio. Historically, the company is best known for their steam boilers. Background The company was founded in 1867 in Providence, Rhode Island, by partners Stephen Wilcox and George Herman Babcock, George Babcock to manufacture and market Wilcox's patented water-tube boiler. B&W's list of innovations and firsts include the world's first installed utility boiler (1881); manufacture of boilers to power New York City's first subway (1902); first pulverized coal power plant (1918); design and manufacture of components for , the world's first nuclear-powered submarine (1953–55); the first supercritical boiler, supercritical pressure coal-fired boiler (1957); design and supply of reactors for the first U.S. built nuclear-powered surface ship, (1961).''Steam/its generation and use'', 41st Edition Histo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleus) to ammonia (). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by Organic compound, organic or other groups (indicated by R). Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it. Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted–Lowry acid–base theory, Brønsted acids (proton donors): : The ammonium ion is mildly acidic, reacting with Brønsted bases to return ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Trioxide
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3. It has been described as "unquestionably the most conomicallyimportant sulfur oxide". It is prepared on an industrial scale as a precursor to sulfuric acid. Sulfur trioxide exists in several forms: gaseous monomer, crystalline trimer, and solid polymer. Sulfur trioxide is a solid at just below room temperature with a relatively narrow liquid range. Gaseous SO3 is the primary precursor to acid rain. Molecular structure and bonding Monomer The molecule SO3 is trigonal planar. As predicted by VSEPR theory, its structure belongs to the D3h point group. The sulfur atom has an oxidation state of +6 and may be assigned a formal charge value as low as 0 (if all three sulfur-oxygen bonds are assumed to be double bonds) or as high as +2 (if the Octet Rule is assumed). When the formal charge is non-zero, the S-O bonding is assumed to be delocalized. In any case the three S-O bond leng ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Oxide
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Charge-neutral *Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide * Nitrogen dioxide (), nitrogen(IV) oxide * Nitrogen trioxide (), or nitrate radical *Nitrous oxide (), nitrogen(0,II) oxide * Dinitrogen dioxide (), nitrogen(II) oxide dimer * Dinitrogen trioxide (), nitrogen(II,IV) oxide * Dinitrogen tetroxide (), nitrogen(IV) oxide dimer *Dinitrogen pentoxide (), nitrogen(V) oxide, or nitronium nitrate * Nitrosyl azide (), nitrogen(−I,0,I,II) oxide * Nitryl azide () * Oxatetrazole () * Trinitramide ( or ), nitrogen(0,IV) oxide Anions Cations * Nitrosonium ( or ) * Nitronium ( or ) Atmospheric sciences In atmospheric chemistry: * (or NO''x'') refers to the sum of NO and . * (or NO''y'') refers to the sum of and all oxidized atmospheric odd-nitrogen species (''e.g.'' the sum of , , , etc.) * (or NO''z'') = − * Mixed Oxides of Nitrogen ("MON"): solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Power Plant
A power station, also referred to as a power plant and sometimes generating station or generating plant, is an industrial facility for the electricity generation, generation of electric power. Power stations are generally connected to an electrical grid. Many power stations contain one or more Electric generator, generators, rotating machine that converts mechanical power into three-phase electric power. The relative motion between a magnetic field and a Electrical conductor, conductor creates an electric current. The energy source harnessed to turn the generator varies widely. Most power stations in the world burn fossil fuels such as coal, petroleum, oil, and natural gas to generate electricity. Low-carbon power sources include nuclear power, and use of renewable energy, renewables such as solar power, solar, wind power, wind, geothermal power, geothermal, and hydroelectricity, hydroelectric. History In early 1871 Belgian inventor Zénobe Gramme invented a generator powerfu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |