|
Quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivatives of aromatic compounds an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,4-Benzoquinone
1,4-Benzoquinone, commonly known as ''para''-quinone, is a chemical compound with the chemical formula, formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated carbonyl compound, α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound. Preparation 1,4-Benzoquinone is prepared industrially by oxidation of hydroquinone, which can be obtained by several routes. One route involves oxidation of Diisopropylbenzenes, diisopropylbenzene and the Hock rearrangem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthraquinone
Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic hydrocarbon, aromatic organic compound with formula . Several isomers exist but these terms usually refer to 9,10-anthraquinone (IUPAC: 9,10-dioxoanthracene) wherein the ketone, keto groups are located on the central ring. It is used as a digester additive to Pulp (paper), wood pulp for papermaking. Many Anthraquinones, anthraquinone derivatives are generated by organisms or synthesised industrially for use as Anthraquinone dyes, dyes, pharmaceuticals, and Catalysis, catalysts. Anthraquinone is a yellow, highly crystalline solid, poorly solubility, soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite. Synthesis There are several current industrial methods to produce 9,10-anthraquinone: # The oxidation of anthracene. Chromium(VI) is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,4-naphthoquinone
1,4-Naphthoquinone or para-naphthoquinone is a quinone derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone. Preparation The industrial synthesis involves aerobic oxidation of naphthalene over a vanadium oxide catalyst: :CH + 3/2 O → CHO + HO In the laboratory, naphthoquinone can be produced by the oxidation of a variety of naphthalene compounds. An inexpensive route involves oxidation of naphthalene with chromium trioxide. Reactions 1,4-Naphthoquinone acts as strong dienophile in Diels-Alder reaction. Its adduct with 1,3-butadiene can be prepared by two methods: 1) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-benzoquinone
1,2-Benzoquinone, also called ''ortho''-benzoquinone, is an organic compound with formula . It is one of the two isomers of quinone, the other being 1,4-benzoquinone. It is a red volatile solid that is soluble in water and diethyl ether. It is rarely encountered because of its instability, but it is of fundamental interest as the parent compound of many derivatives which are known. Structure The molecule has C symmetry. X-ray crystallography shows that the double bonds are localized, with alternatingly long and short C-C distances within the ring. The C=O distances of 1.21 Å are characteristic of ketones. Preparation and reactions 1,2-Benzoquinone is produced on oxidation of catechol exposed to air in aqueous solution or by ortho oxidation of a phenol. A strain of the bacterium ''Pseudomonas mendocina'' metabolises benzoic acid, yielding 1,2-benzoquinone via catechol. Ortho-quinones are widely used in organic synthesis. Occurrence of ''ortho''-quinones 4,5-Dimethyl-1,2-ben ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
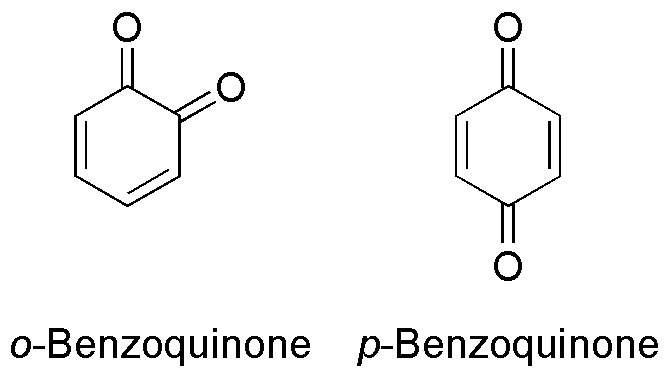
Benzoquinone
Benzoquinone (C6H4O2) is a quinone with a single benzene ring. There are 2 (out of 3 hypothetical) benzoquinones: * 1,4-Benzoquinone, most commonly, right image (also ''para''-benzoquinone, ''p''-benzoquinone, ''para''-quinone, or just quinone) * 1,2-Benzoquinone, less commonly, left image (also ''ortho''-benzoquinone, ''o''-benzoquinone, ''ortho''-quinone) *1,3-benzoquinone "does not exist, because its structure would be nonplanar and highly strained", though derivatives are known. An alkylated ''p''-benzoquinone has been found in the rhizomes of '' Iris kemaonensis''. See also * Arene substitution pattern Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon. ''Ortho'', ''meta'', and ''para'' substitution * ... References {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alizarin
Alizarin (also known as 1,2-dihydroxyanthraquinone, Mordant Red 11, C.I. 58000, and Turkey Red) is an organic compound with formula that has been used throughout history as a red dye, principally for dyeing textile fabrics. Historically it was derived from the roots of plants of the madder genus.The primary madder species from which alizarin historically has been obtained is '' Rubia tinctorum''. See also In 1869, it became the first natural dye to be produced synthetically. Alizarin is the main ingredient for the manufacture of the madder lake pigments known to painters as rose madder and alizarin crimson. Alizarin in the most common usage of the term has a deep red color, but the term is also part of the name for several related non-red dyes, such as Alizarine Cyanine Green and Alizarine Brilliant Blue. A use of alizarin in modern times is as a staining agent in biological research because it stains free calcium and certain calcium compounds a red or light purple color. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols, and steroid ketones. DDQ decomposes in water, but is stable in aqueous mineral acid. Preparation Synthesis of DDQ involves cyanation of chloranil. J. Thiele and F. Günther first reported a 6-step preparation in 1906. The substance did not receive interest until its potential as a dehydrogenation agent was discovered. A single-step chlorination from 2,3-dicyanohydroquinone was reported in 1965. Reactions The reagent removes pairs of H atoms from organic molecules. The stoichiometry of its action is illustrated by the conversion of tetralin to naphthalene: :2 C6Cl2(CN)2O2 + C10H12 → 2 C6Cl2(CN)2(OH)2 + C10H8 The resulting hydroquinone is poorly soluble in typical reaction solvents (dioxane, benzene, alkanes), which facilitates workup. Solutions of DDQ in benzene are red, due to the formation of a charge-t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lawsone
Lawsone (2-hydroxy-1,4-naphthoquinone), also known as hennotannic acid, is a red-orange dye present in the leaves of the henna plant ('' Lawsonia inermis''), for which it is named, as well as in the common walnut ('' Juglans regia'') and water hyacinth ('' Pontederia crassipes''). Humans have used henna extracts containing lawsone as hair and skin dyes for more than 5,000 years. Lawsone reacts chemically with the protein keratin in skin and hair via a Michael addition reaction, resulting in a strong permanent stain that lasts until the skin or hair is shed. Darker colored staining is due to more lawsone–keratin interactions occurring, which evidently break down as the concentration of lawsone decreases and the tattoo fades. Lawsone strongly absorbs UV light, and aqueous extracts can be effective sunless tanning agents and sunscreens. Lawsone is a 1,4-naphthoquinone derivative, an analog of hydroxyquinone containing one additional ring. Lawsone isolation from ''Lawsonia ine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |