|
Potassium Sulfide
Potassium sulfide is an inorganic compound with the formula K2 S. The colourless solid is rarely encountered, because it reacts readily with water, a reaction that affords potassium hydrosulfide (KSH) and potassium hydroxide (KOH). Most commonly, the term potassium sulfide refers loosely to this mixture, not the anhydrous solid. Structure It adopts an antifluorite structure, which means that the small K+ ions occupy the tetrahedral (F−) sites in fluorite, and the larger S2− centers occupy the eight-coordinate sites. Li2S, Na2S, and Rb2S crystallize similarly.Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. . Synthesis and reactions It can be produced by heating K2SO4 with carbon ( coke): :K2SO4 + 4 C → K2S + 4 CO In the laboratory, pure K2S may be prepared by the reaction of potassium and sulfur in anhydrous ammonia. Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. Hydrogen sulfide is toxic to humans and most other animals by inhibiting cellular respiration in a manner similar to hydrogen cyanide. When it is inhaled or its salts are ingested in high amounts, damage to organs occurs rapidly with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the human body produces small amounts of this sulfide and its mineral salts, and uses it as a signalling molecule. Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers; this process is commonly known as anaerobic digestio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Senko Hanabi
Senko hanabi (線香花火 ''senkō hanabi'', literally: incense-stick fireworks) is a traditional Japanese firework. It is a type of sparkler. Essays about it date back to at least 1927. It is a thin shaft of twisted tissue paper about 20 centimeters long with one end containing a few grains of black powder (gunpowder). Black powder consists of three chemicals: potassium nitrate, sulfur, and charcoal. Usage To properly ignite a senko hanabi, the pointed end (with black powder) is held straight down and lit, so that the flame is at the bottom. After a few seconds, a glowing, molten slag ball will form. The slag is reportedly potassium sulfide, which also contains carbon from the charcoal. After a while, the molten ball will initiate the second phase of the firework, silently spraying an array of delicate branching sparks with a range of up to . It is best ignited away from the wind and held with a steady hand, so that the delicate molten ball does not drop and that the two ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Black Powder
Gunpowder, also commonly known as black powder to distinguish it from modern smokeless powder, is the earliest known chemical explosive. It consists of a mixture of sulfur, charcoal (which is mostly carbon), and potassium nitrate, potassium nitrate (saltpeter). The sulfur and charcoal act as fuels while the saltpeter is an oxidizer. Gunpowder has been widely used as a propellant in firearms, artillery, rocketry, and pyrotechnics, including use as a blasting agent for explosives in quarrying, mining, building Pipeline transport, pipelines, tunnels, and road#Construction, roads. Gunpowder is classified as a Explosive#Low, low explosive because of its relatively slow decomposition rate, low ignition temperature and consequently low brisance, brisance (breaking/shattering). Low explosives deflagration, deflagrate (i.e., burn at subsonic speeds), whereas high explosives detonation, detonate, producing a supersonic shockwave. Ignition of gunpowder packed behind a projectile generates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coke (fuel)
Coke is a grey, hard, and porous coal-based fuel with a high carbon content. It is made by heating coal or petroleum in the absence of air. Coke is an important industrial product, used mainly in iron ore smelting, but also as a fuel in stoves and forges. The unqualified term "coke" usually refers to the product derived from low-ash and low-sulphur bituminous coal by a process called coking. A similar product called petroleum coke, or pet coke, is obtained from crude petroleum in oil refinery, petroleum refineries. Coke may also be formed naturally by geology, geologic processes.B. Kwiecińska and H. I. Petersen (2004): "Graphite, semi-graphite, natural coke, and natural char classification — ICCP system". ''International Journal of Coal Geology'', volume 57, issue 2, pages 99-116. It is the residue of a destructive distillation process. Production Industrial coke furnaces The industrial production of coke from coal is called coking. The coal is baked in an airless k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbothermic Reaction
Carbothermic reactions involve the reduction of substances, often metal oxides (O2-), using carbon (C) as the reducing agent. The reduction is usually conducted in the electric arc furnace or reverberatory furnace, depending on the metal ore. These chemical reactions are usually conducted at temperatures of several hundred degrees Celsius. Such processes are applied for production of the elemental forms of many elements. The ability of metals to participate in carbothermic reactions can be predicted from Ellingham diagrams. Carbothermal reactions produce carbon monoxide (CO) and sometimes carbon dioxide (CO2). The facility of these conversions is attributable to the entropy of reaction: two solids, the metal oxide (and flux) and carbon, are converted to a new solid (metal) and a gas (CO), the latter having high entropy. Applications A prominent example is that of iron ore smelting. Many reactions are involved, but the simplified equation is usually shown as: : 2 + 3 C � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Sulfide
Sodium sulfide is a chemical compound with the formula Na2 S, or more commonly its hydrate Na2S·9 H2O. Both the anhydrous and the hydrated salts are colorless solids, although technical grades of sodium sulfide are generally yellow to brick red owing to the presence of polysulfides. It is commonly supplied as a crystalline mass, in flake form, or as a fused solid. They are water-soluble, giving strongly alkaline solutions. When exposed to moisture, Na2S immediately hydrates to give sodium hydrosulfide. Sodium sulfide has an unpleasant rotten egg smell due to the hydrolysis to hydrogen sulfide in moist air. Some commercial samples are described as Na2S·''x''H2O, where a weight percentage of Na2S is specified. Commonly available grades have around 60% Na2S by weight, which means that ''x'' is around 3. These grades of sodium sulfide are often marketed as 'sodium sulfide flakes'. These samples consist of NaSH, NaOH, and water. Structure The structures of sodium sulfides have bee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Sulfide
Lithium sulfide is the inorganic compound with the formula Li2 S. It crystallizes in the antifluorite motif, described as the salt (Li+)2S2−. It forms a solid yellow-white deliquescent powder. In air, it easily hydrolyses to release foul smelling hydrogen sulfide gas. Preparation Lithium sulfide is prepared by treating lithium with sulfur. This reaction is conveniently conducted in anhydrous ammonia. :2 Li + S → Li2S The THF-soluble triethylborane Triethylborane (TEB), also called triethylboron, is an organoborane (a compound with a B–C bond). It is a colorless pyrophoric liquid. Its chemical formula is or , abbreviated . It is soluble in organic solvents tetrahydrofuran and hexane. ... adduct of lithium sulfide can be generated using superhydride. Reactions and applications Lithium sulfide has been considered for use in lithium–sulfur batteries. References External links Lithium Sulfide {{Sulfides Lithium salts Sulfides Fluorite crystal structure< ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
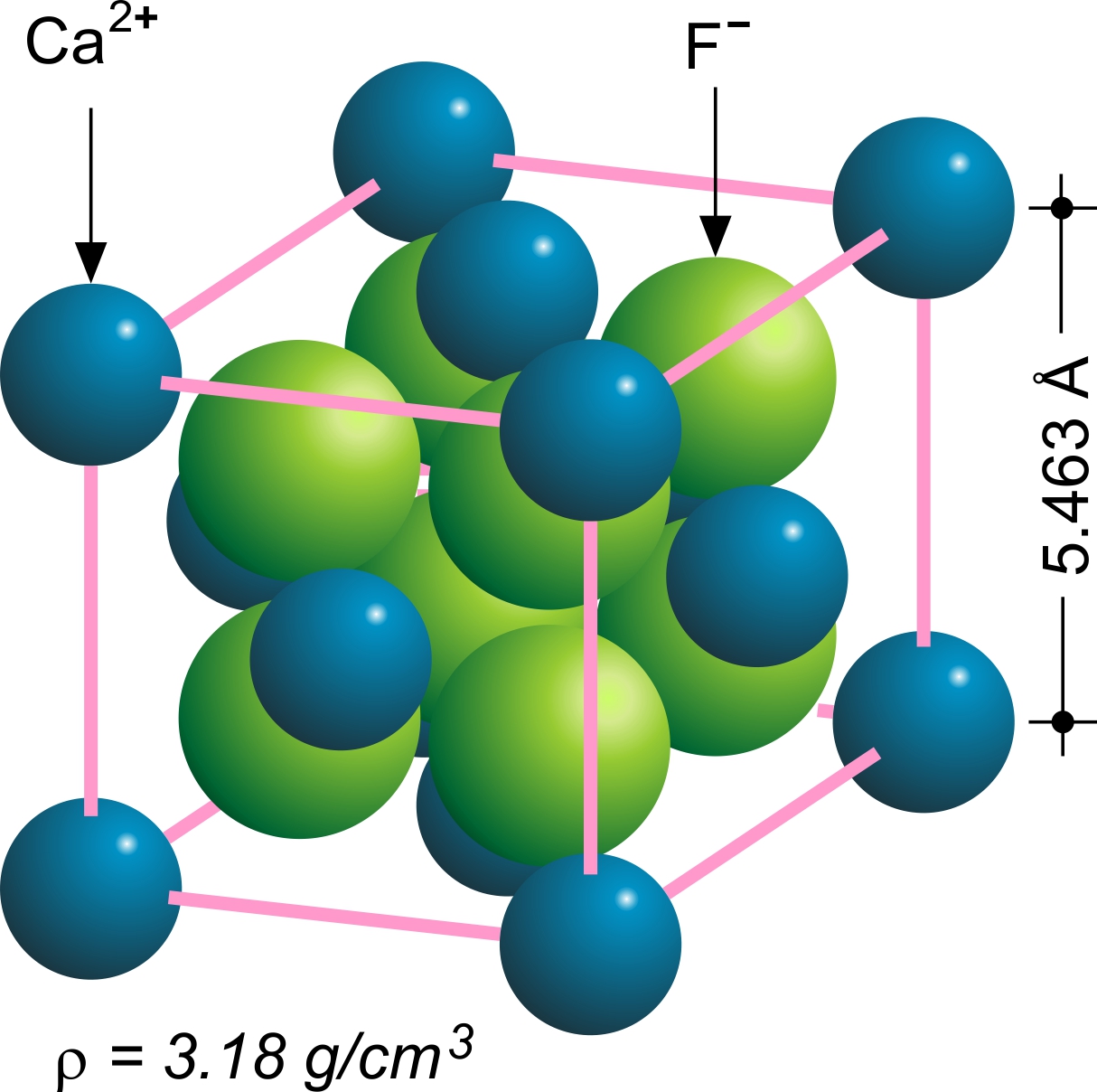
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite has anomalous partial dispersion, that is, its refractive index varies with the wavelength of light in a manner that differs from that of commonly used glasses, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antifluorite Structure
The fluorite structure refers to a common motif for compounds with the formula MX2. The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic (FCC) structure. Many compounds, notably the common mineral fluorite (CaF2), adopt this structure. Many compounds with formula M2X have an antifluorite structure. In these the locations of the anions and cations are reversed relative to fluorite (an anti-structure); the anions occupy the FCC regular sites whereas the cations occupy the tetrahedral interstitial sites. For example, magnesium silicide, Mg2Si, has a lattice parameter of 6.338 Å with magnesium cations occupying the tetrahedral interstitial sites, in which each silicide anion is surrounded by eight magnesium cations and each magnesium cation is surrounded by four silicide anions in a tetrahedral fashion. File:Fluorite Structure.jpg, The fluorite structure of calcium fluoride CaF2. File:Antifluorite Structure.jpg, T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hydrosulfide
Potassium hydrosulfide is an inorganic compound with the formula KSH. This colourless salt consists of the cation and the bisulfide anion . It is the product of the half-neutralization of hydrogen sulfide with potassium hydroxide. The compound is used in the synthesis of some organosulfur compounds. Aqueous solutions of potassium sulfide consist of a mixture of potassium hydrosulfide and potassium hydroxide. The structure of the potassium hydrosulfide resembles that of potassium chloride. Their structure is however complicated by the non-spherical symmetry of the anion An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...s, but these tumble rapidly in the solid. The addition of sulfur gives dipotassium pentasulfide. Synthesis It is prepared by neutralizing aqueous KOH with . R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |