|
Piperidine
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, typical of amines. The name comes from the genus name '' Piper'', which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson and again, independently, in 1852 by the French chemist Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst: : C5H5N + 3 H2 → C5H10NH Pyridine can also be reduce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperine
Piperine, possibly along with its isomer chavicine, is the compound responsible for the pungency of black pepper and long pepper via activation of TRPV1. It has been used in some forms of traditional medicine. Preparation Extraction Due to its poor solubility in water, piperine is typically extracted from black pepper by using organic solvents like dichloromethane or ethanol. The amount of piperine varies from 1–2% in long pepper, to 5–10% in commercial white and black peppers. Piperine can also be prepared by treating the solvent-free residue from a concentrated alcoholic extract of black pepper with a solution of potassium hydroxide to remove resin (said to contain chavicine, an isomer of piperine). The solution is decanted from the insoluble residue and left to stand overnight in alcohol. During this period, the alkaloid slowly crystallizes from the solution. Chemical synthesis Piperine has been synthesized by the action of piperonoyl chloride on piperidine. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
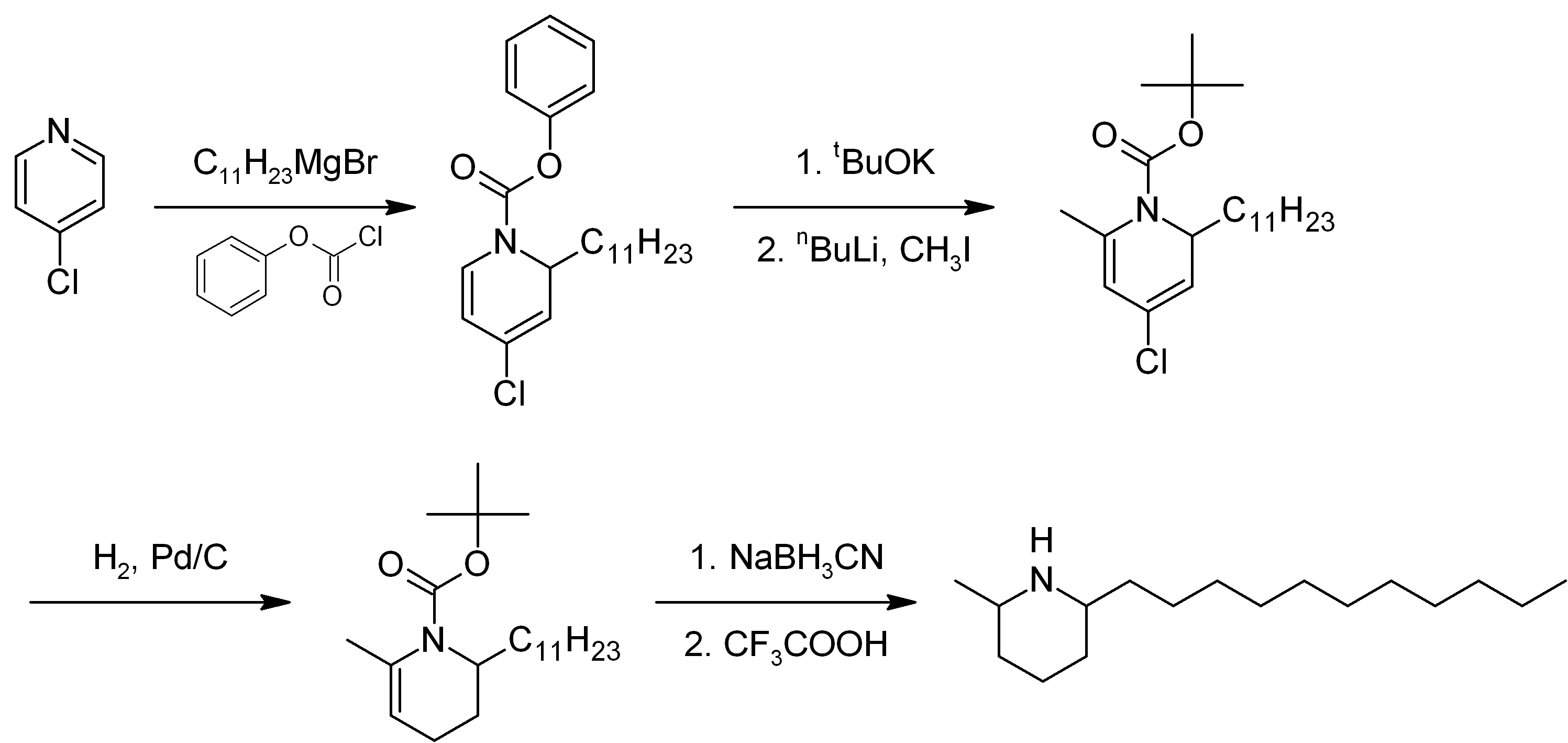
Solenopsin
Solenopsin is a lipophilic alkaloid with the molecular formula C17H35N found in the venom of fire ants (''Solenopsis''). It is considered the primary toxin in the venom and may be the component responsible for the cardiorespiratory failure in people who experience excessive fire ant stings. Structurally solenopsins are a piperidine ring with a methyl group substitution at position 2 and a long hydrophobic chain at position 6. They are typically oily at room temperature, water-insoluble, and present an absorbance peak at 232 nanometers. Fire ant venom contains other chemically related piperidines which make purification of solenopsin from ants difficult. Therefore, solenopsin and related compounds have been the target of organic synthesis from which pure compounds can be produced for individual study. Originally synthesized in 1993, several groups have designed novel and creative methods of synthesizing enantiopure solenopsin and other alkaloidal components of ant venom. To ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated Polymer, polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties Pyridine is diamagnetism, diamagnetic. Its critical point (thermodynamics), critical parameters are: pressure 5.63 MPa, temperature 619 K and volume 248 cm3/mol. In the temperatur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solenopsin
Solenopsin is a lipophilic alkaloid with the molecular formula C17H35N found in the venom of fire ants (''Solenopsis''). It is considered the primary toxin in the venom and may be the component responsible for the cardiorespiratory failure in people who experience excessive fire ant stings. Structurally solenopsins are a piperidine ring with a methyl group substitution at position 2 and a long hydrophobic chain at position 6. They are typically oily at room temperature, water-insoluble, and present an absorbance peak at 232 nanometers. Fire ant venom contains other chemically related piperidines which make purification of solenopsin from ants difficult. Therefore, solenopsin and related compounds have been the target of organic synthesis from which pure compounds can be produced for individual study. Originally synthesized in 1993, several groups have designed novel and creative methods of synthesizing enantiopure solenopsin and other alkaloidal components of ant venom. To ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperazine
Piperazine () is an organic compound with the formula . In term of its structure, it can be described as cyclohexane with the 1- and 4-CH2 groups replaced by NH. Piperazine exists as deliquescent solid with a saline taste. Piperazine is freely soluble in water and ethylene glycol, but poorly soluble in diethyl ether. Piperazine is commonly available industrially is as the hexahydrate, , which melts at 44 °C and boils at 125–130 °C.''The Merck index, 10th Ed.'' (1983), p. 1076, Rahway:Merck & Co. Substituted derivatives of piperazine are a broad class of chemical compounds. Many piperazines have useful pharmacological properties, prominent examples include viagra, ciprofloxacin, and ziprasidone. Origin and naming Piperazines were originally named because of their chemical similarity with piperidine, part of the structure of piperine in the black pepper plant (''Piper nigrum''). The -az- infix added to "piperazine" refers to the extra nitrogen atom, compared to piperidine. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids. Alkaloids are produced by a large variety of organisms including bacteria, fungus, fungi, Medicinal plant, plants, and animals. They can be purified from crude extracts of these organisms by acid-base extraction, or solvent extractions followed by silica-gel column chromatography. Alkaloids have a wide range of pharmacology, pharmacological activities including antimalarial medication, antimalarial (e.g. quinine), asthma, antiasthma (e.g. ephedrine), chemotherapy, anticancer (e.g. omacetaxine mepesuccinate, homoharringtonine), cholinomimetic (e.g. galantamine), vasodilation, vasodilatory (e.g. vincamine), Antiarrhythmic agent, antiarrhythmic (e.g. quinidine), analgesic (e.g. morphine), antibacterial (e.g. chelerythrine), and anti-diabetic, antihyperglycemic activities (e.g. berb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of organic heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of py ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piper (genus)
''Piper'', the pepper plants or pepper vines, is an economically and ecologically important genus in the family (biology), family Piperaceae. It contains about 1,000–2,000 species of shrubs, herbs, and lianas, many of which are dominant species in their native habitat. The diversification of this taxon is of interest to understanding the evolution of plants. Pepper plants belong to the magnoliids, which are angiosperms but neither monocots nor eudicots. Their family (biology), family, Piperaceae, is most closely related to the lizardtail family (Saururaceae), which in fact generally look like smaller, more delicate and wikt:Special:Search/amphibious, amphibious pepper plants. Both families have characteristic tail-shaped inflorescences covered in tiny flowers. A somewhat less close relative is the pipevine family (Aristolochiaceae). A well-known and very close relative – being also part of the Piperaceae – are the radiator plants of the genus ''Peperomia''. The scientific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fire Ant
Fire ants are several species of ants in the genus ''Solenopsis'', which includes over 200 species. ''Solenopsis'' are stinging ants, and most of their common names reflect this, for example, ginger ants and tropical fire ants. Many of the names shared by this genus are often used interchangeably to refer to other species of ant, such as the term red ant, mostly because of their similar coloration despite not being in the genus ''Solenopsis''. Both '' Myrmica rubra'' and '' Pogonomyrmex barbatus'' are common examples of non-Solenopsis ants being termed red ants. None of these common names apply to all species of ''Solenopsis'' nor exclusively to species of ''Solenopsis''; for example, several species of weaver ants of the genus '' Oecophylla'' in Southeast Asia are colloquially called "fire ants" because of their similar coloration and painful bites, but the two genera are not closely related. '' Wasmannia auropunctata'' is another unrelated ant more commonly called the "littl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Black Pepper
Black pepper (''Piper nigrum'') is a flowering vine in the family Piperaceae, cultivated for its fruit (the peppercorn), which is usually dried and used as a spice and seasoning. The fruit is a drupe (stonefruit) which is about in diameter (fresh and fully mature), dark red, and contains a stone which encloses a single pepper seed. Peppercorns and the ground pepper derived from them may be described simply as ''pepper'', or more precisely as ''black pepper'' (cooked and dried unripe fruit), ''green pepper'' (dried unripe fruit), or ''white pepper'' (ripe fruit seeds). Black pepper is native to the Malabar Coast of India, and the Malabar pepper is extensively cultivated there and in other tropical regions. Ground, dried, and cooked peppercorns have been used since antiquity, both for flavour and as a traditional medicine. Black pepper is the world's most traded spice, and is one of the most common spices added to cuisines around the world. Its spiciness is due to the che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |