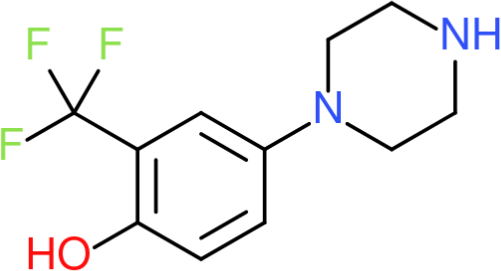|
Phenylpiperazine
1-Phenylpiperazine (1-PP or PP) is a simple chemical compound and drug featuring a phenyl group bound to a piperazine ring. The suffix ‘-piprazole’ is sometimes used in the names of drugs to indicate they belong to this class. It is a rigid analogue of amphetamine. Similarly to amphetamine, 1-PP is a monoamine releasing agent, with values for monoamine release of 186nM for norepinephrine, 880nM for serotonin, and 2,530nM for dopamine. Based on the preceding values, it is about 4.7-fold less potent in releasing serotonin than norepinephrine and about 13.6-fold less potent in releasing dopamine than norepinephrine. Hence, 1-PP is a modestly selective norepinephrine releasing agent (NRA), or could alternatively be thought of as an imbalanced serotonin–norepinephrine releasing agent (SNRA) or serotonin–norepinephrine–dopamine releasing agent (SNDRA). Other homologues and rigid analogues of amphetamine besides 1-PP include 2-aminotetralin (2-AT), 2-amino-1,2-dihydrona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Piperazine
Substituted piperazines are a class of chemical compounds based on a piperazine core. Some are used as recreational drugs and some are used in scientific research. List of substituted piperazines Benzylpiperazines File:Benzylpiperazine.svg, 1-Benzylpiperazine (BZP) File:Methylbenzylpiperazine.svg, 1-Methyl-4-benzylpiperazine (MBZP) File:DBZP.svg, 1,4-Dibenzylpiperazine (DBZP) File:MDBZP.svg, 3,4-Methylenedioxy-1-benzylpiperazine (MDBZP) File:2C-B-BZP.svg, 4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP) File:Methoxypiperamide.png, Methoxypiperamide (MeOP, MEXP) ((4-methoxyphenyl)(4-methylpiperazin-1-yl)methanone) File:Sunifiram.svg , Sunifiram (1-benzoyl-4-propanoylpiperazine) File:3-Methylbenzylpiperazine structure.png, 3-Methylbenzylpiperazine (3-MeBZP) File:Befuraline.svg, Befuraline(also produces benzylpiperazine as a metabolite) File:Fipexide.svg, Fipexide(also produces substituted benzylpiperazine as a metabolite) File:Piberaline.svg, Piberaline(also produces benzy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meta-chlorophenylpiperazine
''meta''-Chlorophenylpiperazine (mCPP) is a psychoactive drug of the phenylpiperazine class. It was initially developed in the late-1970s and used in scientific research before being sold as a designer drug in the mid-2000s. It has been detected in pills touted as legal alternatives to illicit stimulants in New Zealand and pills sold as " ecstasy" in Europe and the United States. Despite its advertisement as a recreational substance, mCPP is actually generally considered to be an unpleasant experience and is not desired by drug users. It lacks any reinforcing effects, but has "psychostimulant, anxiety-provoking, and hallucinogenic effects." It is also known to produce dysphoric, depressive, and anxiogenic effects in rodents and humans, and can induce panic attacks in individuals susceptible to them. It also worsens obsessive–compulsive symptoms in people with the disorder. mCPP is known to induce headaches in humans and has been used for testing potential antimigraine m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Releasing Agent
A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of one or more monoamine neurotransmitters from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitters and hence enhanced signaling by those neurotransmitters. The monoamine neurotransmitters include serotonin, norepinephrine, and dopamine; MRAs can induce the release of one or more of these neurotransmitters. MRAs work by reversing the direction of the monoamine transporters (MATs), including the serotonin transporter (SERT), norepinephrine transporter (NET), and/or dopamine transporter (DAT), causing them to promote efflux of non-vesicular cytoplasmic monoamine neurotransmitter rather than reuptake of synaptic monoamine neurotransmitter. Many, but not all MRAs, also reverse the direction of the vesicular monoamine transporter 2 (VMAT2), thereby additionally resulting in efflux of vesicular monoamine neuro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Para-Methoxyphenylpiperazine
''para''-Methoxyphenylpiperazine (pMeOPP), also known as 4-methoxyphenylpiperazine (4-MeOPP), is a substituted piperazine derivative with stimulant effects which has been sold as an ingredient in "Party pills", initially in New Zealand and subsequently in other countries around the world. Pharmacology pMeOPP is anecdotally said to induce significantly less anxiety than similar piperazines, and is usually taken at doses between 120–200 mg. However it is often mixed with stimulant piperazine derivatives such as benzylpiperazine (BZP) for a combined effect. pMeOPP has been found ''in vitro'' to inhibit the reuptake and induce the release of the monoamine neurotransmitters. This is a mechanism of action shared with drugs of abuse such as amphetamines, and pMeOPP produces somewhat similar effects although it is much less potent and is thought to have relatively insignificant abuse potential. Piperazine derivatives such as trifluoromethylphenylpiperazine (TFMPP) have also been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoromethylphenylpiperazine
3-Trifluoromethylphenylpiperazine (TFMPP) is a recreational drug of the phenylpiperazine chemical class and is a substituted piperazine. Usually in combination with benzylpiperazine (BZP) and other analogues, it is sold as an alternative to the illicit drug MDMA ("Ecstasy"). Use and effects TFMPP is rarely used by itself. In fact, TFMPP reduces locomotor activity and produces aversive effects in animals rather than self-administration, which may explain the decision of the DEA not to permanently make TFMPP a controlled substance. More commonly, TFMPP is co-administered with BZP, which acts as a norepinephrine and dopamine releasing agent. Due to the serotonin agonist effects and increase in serotonin, norepinephrine, and dopamine levels produced by the BZP/TFMPP combination, this mixture of drugs produces effects which crudely mimic those of MDMA. In a clinical study, TFMPP produced effects in humans including dysphoria, dextroamphetamine-like effects (i.e., stimulant-lik ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Antagonist And Reuptake Inhibitor
Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds. List of SARIs Marketed Commercially available serotonin antagonist and reuptake inhibitors include etoperidone (Axiomin, Etonin), lorpiprazole (Normarex), mepiprazole (Psigodal), nefazodone, utility complicated by life-threatening idiosyncratic hepatotoxicity (Serzone, Nefadar), and trazodone (Desyrel). Never marketed * lubazodone (YM-992, YM-35995) – a SARI that, as of this date, had not come to market. Miscellaneous * vilazodone (Viibryd) – a related drug not fitting into this class, as it acts solely as a 5-HT1A receptor partial a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzylpiperazine
Benzylpiperazine (BZP) is a substance often used as a recreational drug and is known to have euphoriant and stimulant properties. Several studies conducted between 2000 and 2011 found that the effects of BZP are similar to amphetamine, although BZP's dosage is roughly 10 times higher by weight. Adverse effects have been reported following its use including psychosis, acute psychosis, renal toxicity and seizures. Deaths from piperazine derivatives are extremely rare, but there has been at least one death apparently due to BZP alone. Its sale is banned in several countries, including Australia, Canada, New Zealand, the United States, the Republic of Ireland, the United Kingdom, Bulgaria, Romania and other parts of Europe. History Development history BZP was first synthesized by Burroughs Wellcome & Company in 1944. It is often claimed that it was originally synthesized as a potential antihelminthic (anti-parasitic) agent for use in farm animals, but its synthesis is thought ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperazine
Piperazine () is an organic compound with the formula . In term of its structure, it can be described as cyclohexane with the 1- and 4-CH2 groups replaced by NH. Piperazine exists as deliquescent solid with a saline taste. Piperazine is freely soluble in water and ethylene glycol, but poorly soluble in diethyl ether. Piperazine is commonly available industrially is as the hexahydrate, , which melts at 44 °C and boils at 125–130 °C.''The Merck index, 10th Ed.'' (1983), p. 1076, Rahway:Merck & Co. Substituted derivatives of piperazine are a broad class of chemical compounds. Many piperazines have useful pharmacological properties, prominent examples include viagra, ciprofloxacin, and ziprasidone. Origin and naming Piperazines were originally named because of their chemical similarity with piperidine, part of the structure of piperine in the black pepper plant (''Piper nigrum''). The -az- infix added to "piperazine" refers to the extra nitrogen atom, compared to piperidine. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-aminotetralin
2-Aminotetralin (2-AT), also known as 1,2,3,4-tetrahydronaphthalen-2-amine (THN), is a stimulant drug with a chemical structure consisting of a tetralin core with an amine as substituent. 2-AT is a rigid analogue of phenylisobutylamine and fully substitutes for d-amphetamine in rat drug discrimination tests, although at one-half to one-eighth the potency. It showed greater potency than a variety of other amphetamine homologues, including 2-amino-1,2-dihydronapthalene (2-ADN), 2-aminoindane (2-AI), 1-naphthylaminopropane (1-NAP), 2-naphthylaminopropane (2-NAP), 1-phenylpiperazine (1-PP), , and . 2-AT has been shown to inhibit the reuptake of serotonin and norepinephrine, and might induce their release as well. It is also likely to act on dopamine on account of its full substitution of d-amphetamine in rodent studies. Chemical derivatives A number of derivatives of 2-aminotetralin exist, including: See also * 1-Aminotetralin 1-Aminotetralin (1-AT), also known as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-naphthylaminopropane
Naphthylaminopropane (NAP; code name PAL-287), also known as naphthylisopropylamine (NIPA), is an experimental drug of the amphetamine and naphthylaminopropane families that was under investigation for the treatment of alcohol and stimulant addiction. Pharmacology Pharmacodynamics Activities Naphthylaminopropane is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). Its values for induction of monoamine release are 3.4nM for serotonin, 11.1nM for norepinephrine, and 12.6nM for dopamine. The drug is also an agonist of the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors. Its values are 466nM at the serotonin 5-HT2A receptor, 40nM at the serotonin 5-HT2B receptor, and 2.3nM at the serotonin 5-HT2C receptor. It is a full agonist of the serotonin 5-HT2A and 5-HT2B receptors and a weak partial agonist of the serotonin 5-HT2C receptor ( = 20%). Naphthylaminopropane has been found to act as a potent monoamine oxidase A (MAO-A) inhibitor, with an of 420nM. This is s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Amino-1,2-dihydronaphthalene
2-Amino-1,2-dihydronapthalene (2-ADN or ADN) is a stimulant drug. It is a rigid analogue of phenylisobutylamine and substitutes for amphetamine in rat drug discrimination tests, although at approximately one-fourth the potency. The drug is closely related to 2-aminotetralin (2-AT; 2-amino-1,2,3,4-tetrahydronaphthalene), which also substitutes for amphetamine, and is about twice as potent as 2-AT in substituting for amphetamine. Other homologous and rigid analogues of amphetamine besides 2-ADN and 2-AT include 2-aminoindane (2-AI), 1-naphthylaminopropane (1-NAP), 2-naphthylaminopropane (2-NAP), 1-phenylpiperazine (1-PP), , and . See also * 2-Naphthylamine 2-Naphthylamine or 2-aminonaphthalene is one of two isomeric aminonaphthalenes, compounds with the formula C10H7NH2. It is a colorless solid, but samples take on a reddish color in air because of oxidation. It was formerly used to make azo dyes, b ... References {{DEFAULTSORT:Amino-1,2-dihydronaphthalene, 2- No ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |