|
Phenyl Compounds
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen atom, which may be replaced by some other element or compound to serve as a functional group. A phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, the phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with and is represented by the symbol Ph (archaically, Φ), or Ø. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. Fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English chemist and physicist who contributed to the study of electrochemistry and electromagnetism. His main discoveries include the principles underlying electromagnetic induction, diamagnetism, and electrolysis. Although Faraday received little formal education, as a self-made man, he was one of the most influential scientists in history. It was by his research on the magnetic field around a Electrical conductor, conductor carrying a direct current that Faraday established the concept of the electromagnetic field in physics. Faraday also established that magnetism could Faraday effect, affect rays of light and that there was an underlying relationship between the two phenomena. the 1911 ''Encyclopædia Britannica''. He similarly discovered the principles of electromagnetic induction, diamagnetism, and the Faraday's laws of electrolysis, laws of electrolysis. His inventions of electric motor, electromagnetic rotar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
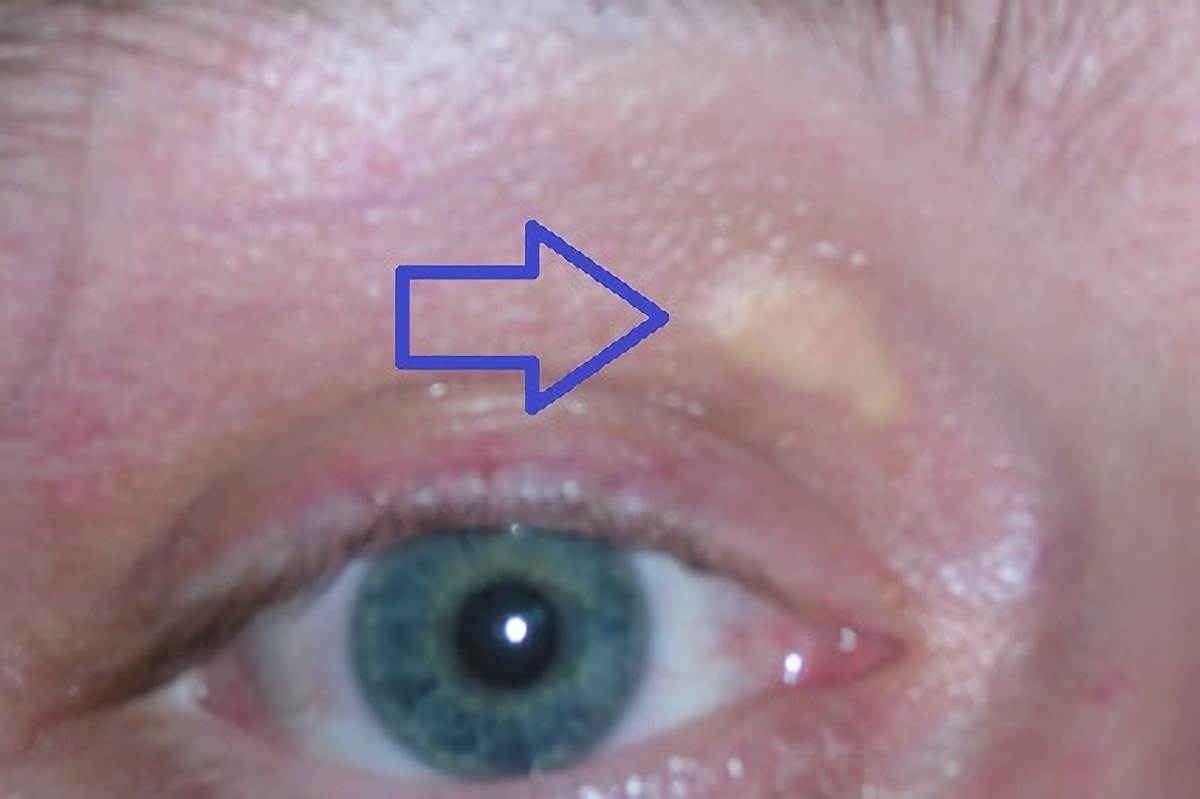
Hypercholesterolaemia
Hypercholesterolemia, also called high cholesterol, is the presence of high levels of cholesterol in the blood. It is a form of hyperlipidemia (high levels of lipids in the blood), hyperlipoproteinemia (high levels of lipoproteins in the blood), and dyslipidemia (any abnormalities of lipid and lipoprotein levels in the blood). Elevated levels of non-HDL cholesterol and LDL in the blood may be a consequence of diet, obesity, inherited (genetic) diseases (such as LDL receptor mutations in familial hypercholesterolemia), or the presence of other diseases such as type 2 diabetes and an underactive thyroid. Cholesterol is one of three major classes of lipids produced and used by all animal cells to form membranes. Plant cells manufacture phytosterols (similar to cholesterol) but in small quantities. Cholesterol is the precursor of the steroid hormones and bile acids. Since cholesterol is insoluble in water, it is transported in the blood plasma within protein particles ( lipop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceutical Drug
Medication (also called medicament, medicine, pharmaceutical drug, medicinal product, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are classified in many ways. One of the key divisions is by level of control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the medical prescription) from over-the-counter drugs (those that consumers can order for themselves). Medicines may be classified by mode of action, route of administration, biological system affected, or therapeutic effects. The World Health Organization keeps a list of essential medicines. Drug discovery and drug development are complex and expensive endeavors undertaken by pharmaceutical companies, academic scientists, and governments. As ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atorvastatin
Atorvastatin, sold under the brand name Lipitor among others, is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a first-line treatment in reducing cholesterol. It is taken by mouth. Common side effects may include diarrhea, heartburn, nausea, muscle pain (typically mild and dose-dependent) and, less frequently, joint pain. Muscle symptoms often occur during the first year and are commonly influenced by pre-existing health issues and the nocebo effect. Most patients can continue therapy with dose adjustment or statin switching. Rare (<0.1%) but serious side effects may include rhabdomyolysis (severe muscle disorder), liver problems and diabetes. Use during may harm the |
Phenylmagnesium Bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It forms colorless crystals. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide is a Grignard reagent. It is often used as a synthetic equivalent for the phenyl "Ph−" synthon. Preparation Phenylmagnesium bromide is commercially available as solutions of diethyl ether or THF. Laboratory preparation involves treating bromobenzene with magnesium metal, usually in the form of turnings. A small amount of iodine may be used to activate the magnesium to initiate the reaction. Coordinating solvents such as ether or THF, are required to solvate (complex) the magnesium(II) center. The solvent must be aprotic since alcohols and water contain an acidic proton and thus react with phenylmagnesium bromide to give benzene. Carbonyl-containing solvents, such as acetone and ethyl acetate, are also incompatible with the reagent. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula . It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses. Crystalline phenyllithium is colorless; however, solutions of phenyllithium are various shades of brown or red depending on the solvent used and the impurities present in the solute. Preparation Phenyllithium was first produced by the reaction of lithium metal with diphenylmercury: : Reaction of a phenyl halide with lithium metal produces phenyllithium: : Phenyllithium can also be synthesized with a metal-halogen exchange reaction: : The predominant method of producing phenyllithium today are the latter two syntheses. Reactions The primary use of PhLi is to facilitate formation of carbon-carbon bonds by nucleophilic addition and substitution reactions: : 2-Phenylpyridine is prepared by the reaction of phenyl lithium with pyridine, a process that entail ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Ring Current
An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring. This is a direct consequence of Ampère's law; since the electrons involved are free to circulate, rather than being localized in bonds as they would be in most non-aromatic molecules, they respond much more strongly to the magnetic field. The ring current creates its own magnetic field. Outside the ring, this field is in the same direction as the externally applied magnetic field; inside the ring, the field counteracts the externally applied field. As a result, the net magnetic field outside the ring is greater than the externally applied field alone, and is less inside the ring. Relevance to NMR spectroscopy Aromatic ring currents are relevant to NMR spectroscopy, as they dramatically influence the chemical shift ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Shift
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a molecule. Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy. Some atomic nuclei possess a magnetic moment (nuclear spin), which gives rise to different energy levels and resonance frequencies in a magnetic field. The total magnetic field experienced by a nucleus includes local magnetic fields induced by currents of electrons in the molecular orbitals (electrons have a magnetic moment themselves). The electron distribution of the same type of nucleus (e.g. ) usually varies according to the local geometry (binding partners, bond lengths, angles between bonds, and so on), and with it the local magnetic field at each nucleus. This is reflected in the spin energy levels (an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Magnetic Resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. High-resolution nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crysta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ångström
The angstrom (; ) is a unit of length equal to m; that is, one ten-billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). It was originally spelled with Swedish letters, as Ångström and later as ångström (). The latter spelling is still listed in some dictionaries, but is now rare in English texts. Some popular US dictionaries list only the spelling ''angstrom''. The unit's symbol is Å, which is a letter of the Swedish alphabet, regardless of how the unit is spelled. However, "A" or "A.U." may be used in less formal contexts or typographically limited media. The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals, wavelengths of electromagnetic radiation, and dimensions of integrated circuit part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Orbital Theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. The MOT explains the paramagnetic nature of O2, which valence bond theory cannot explain. In molecular orbital theory, electrons in a molecule are not assigned to individual chemical bonds between atoms, but are treated as moving under the influence of the atomic nuclei in the whole molecule. Quantum mechanics describes the spatial and energetic properties of electrons as molecular orbitals that surround two or more atoms in a molecule and contain valence electrons between atoms. Molecular orbital theory revolutionized the study of chemical bonding by approximating the states of bonded electrons – the molecular orbitals – as linear combinations of atomic orbitals (LCAO). These approximations are made by applying the density functional theory (DFT) or Hartree–Fock (HF) models to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |