|
Periodyl Fluoride
Periodyl fluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula . The compound has been initially synthesized around 1950. Synthesis Synthesis of periodyl fluoride is by fluorination In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. ... of in liquid HF: : Physical properties Periodyl fluoride forms colorless crystals. Decomposes at 90 to 100 °C. References {{Fluorine compounds Oxyfluorides Iodine compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perchloryl Fluoride
Perchloryl fluoride is a reactive gas with the chemical formula . It has a characteristic sweet odor that resembles gasoline and kerosene. It is toxic and is a powerful oxidizing and fluorinating agent. It is the acid fluoride of perchloric acid. In spite of its small enthalpy of formation (Δf''H''° = ), it is kinetically stable, decomposing only at 400 °C. It is quite reactive towards reducing agents and anions, however, with the chlorine atom acting as an electrophile. It reacts explosively with reducing agents such as metal amides, metals, hydrides, etc. Its hydrolysis in water occurs very slowly, unlike that of chloryl fluoride. Synthesis and chemistry Perchloryl fluoride is produced primarily by the fluorination of perchlorates. The initial syntheses in the early 1950s used fluorine gas or fluorides and anodic oxidation as the fluorinating agents, but these give explosive gaseous mixtures. A common fluorinator in modern syntheses is antimony pentafluoride: : Alt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perbromyl Fluoride
Perbromyl fluoride is an inorganic compound of bromine, fluorine, and oxygen with the chemical formula . Synthesis Synthesis of perbromyl fluoride is by the effect of antimony pentafluoride on a solution of potassium perbromate in hydrofluoric acid: : Physical properties Perbromyl fluoride is a colorless gas at room temperature that is stable in the absence of moisture. Chemical properties Perbromyl fluoride reacts with water to produce perbromic acid and hydrogen fluoride Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...: : References {{Authority control Oxyfluorides Bromine compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep Mantle (geology), mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salt (chemistry), salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within life, living things. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek , meaning 'violet'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compounds, it has also found favour as a non-toxic radiocontrast material. Because of the spec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extremely Reactivity (chemistry), reactive as it reacts with all other Periodic table, elements except for the light Noble gas, noble gases. It is highly toxicity, toxic. Among the elements, fluorine ranks Abundance of the chemical elements, 24th in cosmic abundance and 13th in crustal abundance. Fluorite, the primary mineral source of fluorine, which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Academic Press
Academic Press (AP) is an academic book publisher founded in 1941. It launched a British division in the 1950s. Academic Press was acquired by Harcourt, Brace & World in 1969. Reed Elsevier said in 2000 it would buy Harcourt, a deal completed the next year, after a regulatory review. Thus, Academic Press is now an imprint of Elsevier. Academic Press publishes reference books, serials and online products in the subject areas of: * Communications engineering * Economics * Environmental science * Finance * Food science and nutrition * Geophysics * Life sciences * Mathematics and statistics * Neuroscience * Physical sciences * Psychology Psychology is the scientific study of mind and behavior. Its subject matter includes the behavior of humans and nonhumans, both consciousness, conscious and Unconscious mind, unconscious phenomena, and mental processes such as thoughts, feel ... Well-known products include the '' Methods in Enzymology'' series and encyclopedias such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeitschrift Für Physikalische Chemie
A magazine is a periodical literature, periodical publication, print or digital, produced on a regular schedule, that contains any of a variety of subject-oriented textual and visual content (media), content forms. Magazines are generally financed by advertising, newsagent's shop, purchase price, prepaid subscription business model, subscriptions, or by a combination of the three. They are categorised by their frequency of publication (i.e., as weeklies, monthlies, quarterlies, etc.), their target audiences (e.g., women's and trade magazines), their subjects of focus (e.g., popular science and religious), and their tones or approach (e.g., works of satire or humor). Appearance on the cover of print magazines has historically been understood to convey a place of honor or distinction to an individual or event. Term origin and definition Origin The etymology of the word "magazine" suggests derivation from the Arabic language, Arabic (), the broken plural of () meaning "depot, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorination
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens (). Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for introducing halogens into diverse substrates, e.g. thionyl chloride. Organic chemistry Several pathways exist for the halogenation of organic compounds, including free radical halogenation, ketone halogenation, electrophilic halogenation, and halogen addition reaction. The nature of the substrate determines the pathway. The facility of halogenation is influenced by the halogen. Fluorine and chlorine are more electrophilic and are more aggressive halogenati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Periodate
Potassium periodate is an inorganic salt with the molecular formula KIO4. It is composed of a potassium cation and a periodate anion and may also be regarded as the potassium salt of periodic acid. Note that the pronunciation is per-iodate, not period-ate. Unlike other common periodates, such as sodium periodate and periodic acid, it is only available in the ''meta''periodate form; the corresponding potassium ''ortho''periodate (K5IO6) has never been reported. Preparation Potassium periodate can be prepared by the oxidation of an aqueous solution of potassium iodate by chlorine and potassium hydroxide. : It can also be generated by the electrochemical oxidation of potassium iodate, however the low solubility of KIO3 makes this approach of limited use. Chemical properties Potassium periodate decomposes at 582 °C to form potassium iodate and oxygen. The low solubility of KIO4 makes it useful for the determination of potassium and cerium. M. Venugopalan and K. J. George: "D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
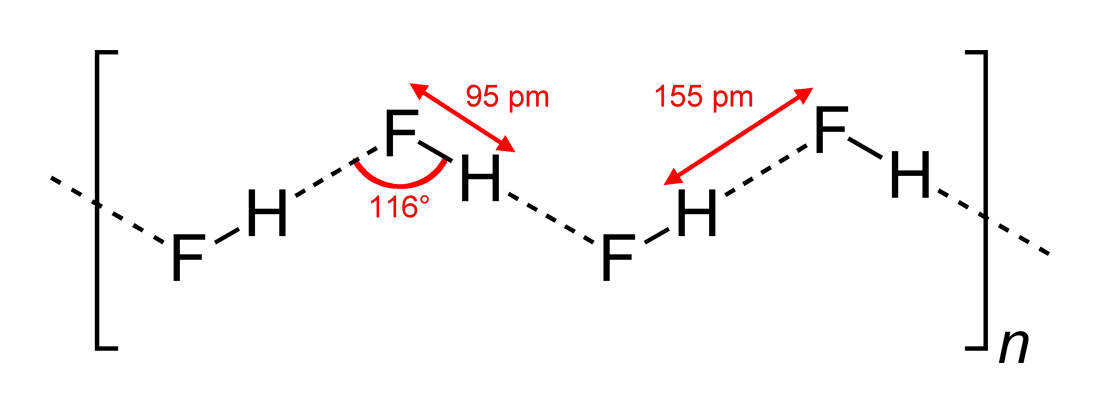
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |