|
Malononitrile
Malononitrile is an organic compound nitrile with the formula . It is a colorless or white solid. It can be prepared by dehydration of cyanoacetamide. Malononitrile is relatively acidic, with a p''K''a of 11 in water. This allows it to be used in the Knoevenagel condensation, for example in the preparation of CS gas: Malononitrile is a suitable starting material for the Gewald reaction, where the nitrile condenses with a ketone or aldehyde in the presence of elemental sulfur and a base to produce a 2-aminothiophene. See also * Malonic acid Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid' ... * Diethyl malonate References External links WebBook page for C3H2N2 {{Authority control Alkanedinitriles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CS Gas
The compound 2-chlorobenzalmalononitrile (also called ''o''-chlorobenzylidene malononitrile; chemical formula: C10H5ClN2), a cyanocarbon, is the defining component of tear gas commonly referred to as CS gas, which is used as a riot control agent. Exposure causes a burning sensation and tearing of the eyes to the extent that the subject cannot keep their eyes open, and a burning irritation of the mucous membranes of the nose, mouth and throat, resulting in profuse coughing, nasal mucus discharge, disorientation, and difficulty breathing, partially incapacitating the subject. CS gas is an aerosol of a volatile solvent (a substance that dissolves other active substances and that easily evaporates) and 2-chlorobenzalmalononitrile, which is a solid compound at room temperature. CS gas is generally accepted as being non-lethal. It was first synthesized by two Americans, Ben Corson and Roger Stoughton, at Middlebury College in 1928, and the chemical's name is derived from the firs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile ( hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The skeleton is linear with a short distance of 1.16 Å. Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas. Applications Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminopropionitrile
Aminopropionitrile, also known as β-aminopropionitrile (BAPN), is an organic compound with both amine and nitrile functional groups. It is a colourless liquid. The compound occurs naturally and is of interest in the biomedical community. Biochemical and medical occurrence BAPN is the toxic constituent of peas from Lathyrus plants, e.g., lathyrus odoratus. Lathyrism, a disease known for centuries, encompasses 2 distinct entities: a disorder of the nervous system (neurolathyrism) leading to limb paralysis, and a disorder of connective tissue, causing either bone deformity (osteolathyrism) or aortic aneurisms (angiolathyrim). BAPN causes osteolathyrism and angiolathyrism when ingested in large quantities." It can cause osteolathyrism, neurolathyrism, and/or angiolathyrism. It is an antirheumatic agent in veterinary medicine. It has attracted interest as an anticancer agent. Production Aminopropionitrile is prepared by the reaction of ammonia with acrylonitrile.Karsten Eller, Erha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethylsuccinonitrile
Tetramethylsuccinonitrile or TMSN is an organic compound with the formula (C(CH3)2CN)2, classified as a dinitrile. It is a colorless and odorless solid. TMSN is the byproduct from the use of some radical initiators used in polymer manufacture. TMSN is derived from 2,2'-azobis-isobutyronitrile: :(NC(CH3)2CN)2 → (C(CH3)2CN)2 + N2 AIBN is a common radical initiator in the manufacture of polyvinyl chloride polymers. Safety considerations Because PVC is pervasive and can contain TMSN, the safety aspects of this dinitrile has generated interest. Symptoms of large or short exposure to this substance include convulsions, dizziness, headache, nausea, vomiting or even unconsciousness, hence affects central nervous system. In regards to occupational exposures, the Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health The National Institute for Occupational Safety and Health (NIOSH, ) is the United States federal agency respons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinonitrile
Succinonitrile, also butanedinitrile, is a nitrile, with the formula of C2H4(CN)2. It is a colorless waxy solid which melts at 58 °C. Succinonitrile is produced by the addition of hydrogen cyanide to acrylonitrile (hydrocyanation): :CH2=CHCN + HCN → NCCH2CH2CN Hydrogenation of succinonitrile yields putrescine (1,4-diaminobutane). See also * Malononitrile - A di-nitrile with 3 carbon atoms * Glutaronitrile - A di-nitrile with 5 carbon atoms * Adiponitrile Adiponitrile is an organic compound with the chemical formula (CH2)4(CN)2. This viscous, colourless dinitrile is an important precursor to the polymer nylon 66. In 2005, about one million tonnes of adiponitrile were produced.M. T. Musser, "Adipi ... - A di-nitrile with 6 carbon atoms References External links WebBook page for C4H4N2 Alkanedinitriles {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyronitrile
Butyronitrile or butanenitrile or propyl cyanide, is a nitrile with the formula C3H7CN. This colorless liquid is miscible with most polar organic solvents. Uses Butyronitrile is mainly used as a precursor to the poultry drug amprolium.Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" ''Ullmann's Encyclopedia of Industrial Chemistry'' 2002, Wiley-VCH, Weinheim. It also has recognized use in the synthesis of Etifelmine. Synthesis Butyronitrile is prepared industrially by the ammoxidation of ''n''-butanol: :C3H7CH2OH + NH3 + O2 → C3H7CN + 3 H2O Occurrence in space Butyronitrile has been detected in the Large Molecule Heimat The Large Molecule Heimat is a dense gas cloud located in the molecular cloud Sagittarius B2. Many species of molecule, including aminoacetonitrile (a molecule related to glycine), ethyl formate Ethyl formate is an ester formed when ethanol (an .... References External links NIST Chemistry WebBook page fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone Cyanohydrin
Acetone cyanohydrin (ACH) is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. It liberates hydrogen cyanide easily, so it is used as a source of such. For this reason, this cyanohydrin is also highly toxic. Preparation In the laboratory, this compound may be prepared by treating sodium cyanide with acetone, followed by acidification: : Considering the high toxicity of acetone cyanohydrin, a lab scale production has been developed using a microreactor-scale flow chemistry to avoid needing to manufacture and store large quantities of the reagent. Alternatively, a simplified procedure involves the action of sodium or potassium cyanide on the sodium bisulfite adduct of acetone prepared ''in situ''. This gives a less pure product, one that is nonetheless suitable for most syntheses. Reactions Acetone cyanohydrin is an intermediate en route to methyl methacrylate. Treatme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pivalonitrile
Pivalonitrile is a nitrile with the semi-structural formula (CH3)3CCN, abbreviated ''t''-BuCN. This aliphatic organic compound is a clear, colourless liquid that is used as a solvent and as a labile ligand in coordination chemistry. Pivalonitrile is isomeric with ''tert''-butyl isocyanide but the two compounds do not exist in chemical equilibrium, unlike its silicon analog trimethylsilyl cyanide Trimethylsilyl cyanide is the chemical compound with the formula (CH3)3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen .... References 5 Tert-butyl compounds {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propanenitrile
Propionitrile, also known as ethyl cyanide and propanenitrile, is an organic compound with the formula CH3CH2CN. It is a simple aliphatic nitrile. The compound is a colourless, water-soluble liquid. It is used as a solvent and a precursor to other organic compounds. Production The main industrial route to this nitrile is the hydrogenation of acrylonitrile. It is also prepared by the ammoxidation of propanol (propionaldehyde can also be used instead):Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. :CH3CH2CH2OH + O2 + NH3 → CH3CH2CN + 3 H2O Propionitrile is a byproduct of the electrodimerisation of acrylonitrile to adiponitrile. In the laboratory propanenitrile can also be produced by the dehydration of propionamide, by catalytic reduction of acrylonitrile, or by distilling ethyl sulfate and potassium cyanide. Applications Propionitrile is a solvent si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanogen
Cyanogen is the chemical compound with the formula ( C N)2. It is a colorless and highly toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C‒ C≡N, although other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide (NCBr) (but see also '' Cyano radical''.) Cyanogen is the anhydride of oxamide: :H2NC(O)C(O)NH2 → NCCN + 2 H2O although oxamide is manufactured from cyanogen by hydrolysis: :NCCN + 2 H2O → H2NC(O)C(O)NH2 Preparation Cyanogen is typically generated from cyanide compounds. One laboratory method entails thermal decomposition of mercuric cyanide: :2 Hg(CN)2 → (CN)2 + Hg2(CN)2 Alternatively, one can combine solutions of copper(II) salts (such as copper(II) s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolonitrile
Glycolonitrile, also called hydroxyacetonitrile or formaldehyde cyanohydrin, is the organic compound with the formula HOCH2CN. It is the simplest cyanohydrin and it is derived from formaldehyde. It is a colourless liquid that dissolves in water and ether. Because glycolonitrile decomposes readily into formaldehyde and hydrogen cyanide, it is listed as an extremely hazardous substance. In January 2019, astronomers reported the detection of glycolonitrile, another possible building block of life among other such molecules, in outer space. Synthesis and reactions Glycolonitrile is produced by reacting formaldehyde with hydrogen cyanide under acidic conditions. This reaction is catalysed by base..Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" ''Ullmann's Encyclopedia of Industrial Chemistry'' 2002, Wiley-VCH, Weinheim. Glycolonitrile polymerizes under alkaline conditions above pH 7.0. As the product of polymerization is an amine with a basic char ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knoevenagel Condensation
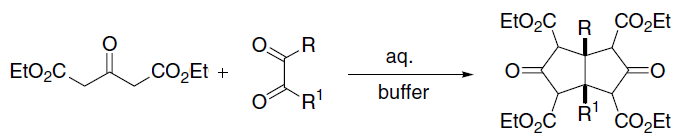
In organic chemistry, the Knoevenagel condensation () reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation. A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence ''condensation''). The product is often an α,β-unsaturated ketone (a conjugated enone). In this reaction the carbonyl group is an aldehyde or a ketone. The catalyst is usually a weakly basic amine. The active hydrogen component has the form * or for instance diethyl malonate, Meldrum's acid, ethyl acetoacetate or malonic acid, or cyanoacetic acid. * , for instance nitromethane. where Z is an electron withdrawing group. Z must be powerful enough to facilitate deprotonation to the enolate ion even with a mild base. Using a strong base in this reaction would induce self-condensation of the aldeh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |