|
Mixture Fraction
Mixture fraction (Z) is a quantity used in combustion studies that measures the mass fraction of one stream of a mixture formed by two feed streams, one the fuel stream and the other the oxidizer stream. Both the feed streams are allowed to have inert gases. The mixture fraction definition is usually normalized such that it approaches unity in the fuel stream and zero in the oxidizer stream. The mixture-fraction variable is commonly used as a replacement for the physical coordinate normal to the flame surface, in nonpremixed combustion. Definition Assume a two-stream problem having one portion of the boundary the fuel stream with fuel mass fraction Y_F=Y_ and another portion of the boundary the oxidizer stream with oxidizer mass fraction Y_=Y_. For example, if the oxidizer stream is air and the fuel stream contains only the fuel, then Y_=0.232 and Y_=1. In addition, assume there is no oxygen in the fuel stream and there is no fuel in the oxidizer stream. Let s be the mass of oxyge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion (e.g., using a lit match to light a fire), the heat from a flame may provide enough energy to make the reaction self-sustaining. The study of combustion is known as combustion science. Combustion is often a complicated sequence of elementary reaction, elementary Radical (chemistry), radical reactions. Solid fuels, such as wood and coal, first undergo endothermic pyrolysis to produce gaseous fuels whose combustion then supplies the heat required to produce more of them. Combustion is often hot e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Fraction (chemistry)
In chemistry, the mass fraction of a substance within a mixture is the ratio w_i (alternatively denoted Y_i) of the mass m_i of that substance to the total mass m_\text of the mixture. Expressed as a formula, the mass fraction is: : w_i = \frac . Because the individual masses of the ingredients of a mixture sum to m_\text, their mass fractions sum to unity: : \sum_^ w_i = 1. Mass fraction can also be expressed, with a denominator of 100, as percentage by mass (in commercial contexts often called ''percentage by weight'', abbreviated ''wt.%'' or ''% w/w''; see mass versus weight). It is one way of expressing the composition of a mixture in a dimensionless size; mole fraction (percentage by moles, mol%) and volume fraction ( percentage by volume, vol%) are others. When the prevalences of interest are those of individual chemical elements, rather than of compounds or other substances, the term ''mass fraction'' can also refer to the ratio of the mass of an element to the tot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkanes
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula . The alkanes range in complexity from the simplest case of methane (), where ''n'' = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like hexacontane () or 4-methyl-5-(1-methylethyl) octane, an isomer of dodecane (). The International Union of Pure and Applied Chemistry (IUPAC) defines alkanes as "acyclic branched or unbranched hydrocarbons having the general formula , and therefore consisting entirely of hydrogen atoms and saturated carbon atoms". However, some sources use the term to denote ''any'' saturated hydrocarbon, including those that are either monocyclic (i.e. the cycloalkanes) or polycycl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equivalence Ratio
Equivalence or Equivalent may refer to: Arts and entertainment *Album-equivalent unit, a measurement unit in the music industry *Equivalence class (music) *'' Equivalent VIII'', or ''The Bricks'', a minimalist sculpture by Carl Andre *'' Equivalents'', a series of photographs of clouds by Alfred Stieglitz Language *Dynamic and formal equivalence in translation * Equivalence (formal languages) Law *The doctrine of equivalents in patent law *The equivalence principle as if impacts on the direct effect of European Union law Logic *Logical equivalence, where two statements are logically equivalent if they have the same logical content * Material equivalence, a relationship where the truth of either one of the connected statements requires the truth of the other Science and technology Chemistry * Equivalent (chemistry) *Equivalence point *Equivalent weight Computing * Turing equivalence (theory of computation), or Turing completeness *Semantic equivalence in computer metadata E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amable Liñán
Amable Liñán Martínez (born 1934 in Noceda de Cabrera, Castrillo de Cabrera, León, Spain) is a Spanish aeronautical engineer working in the field of combustion. Biography He holds a PhD in Aeronautical Engineering from the Technical University of Madrid, advised by Gregorio Millán Barbany and Degree of Aeronautical Engineer from the Caltech advised by Frank E. Marble. He is currently Professor of Fluid Mechanics and professor emeritus at the Higher Technical School of Aeronautical Engineers of the Polytechnic University of Madrid (attached to the Department of Motorcycle and Thermofluidodynamics of said school). He has taught at universities in California, Michigan and Princeton University in the United States and in Marseilles in France, among others. Since 1997 he is an adjunct professor at Yale University. Research He has focused his research studies on the basic problems of combustion, both reactor and planetary probe dynamics, in the latter case working directl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Number
In fluid dynamics and thermodynamics, the Lewis number (denoted ) is a dimensionless number defined as the ratio of thermal diffusivity to mass diffusivity. It is used to characterize fluid flows where there is simultaneous heat and mass transfer. The Lewis number puts the thickness of the thermal boundary layer in relation to the concentration boundary layer. The Lewis number is defined as :\mathrm = \frac = \frac . where: * is the thermal diffusivity, * is the mass diffusivity, * is the thermal conductivity, * is the density, * is the mixture-averaged diffusion coefficient, * is the specific heat capacity at constant pressure. In the field of fluid mechanics, many sources define the Lewis number to be the inverse of the above definition. The Lewis number can also be expressed in terms of the Prandtl number () and the Schmidt number (): :\mathrm = \frac It is named after Warren K. Lewis (1882–1975), who was the first head of the Chemical Engineering Department at MIT. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
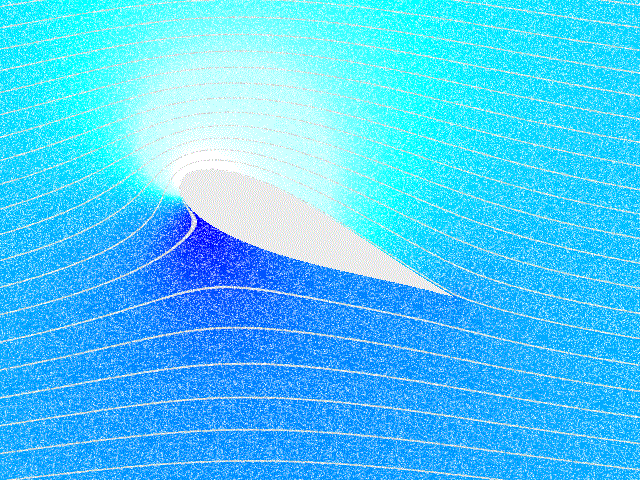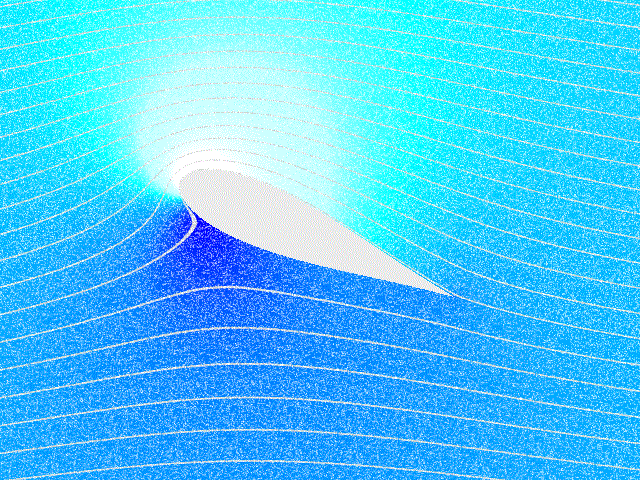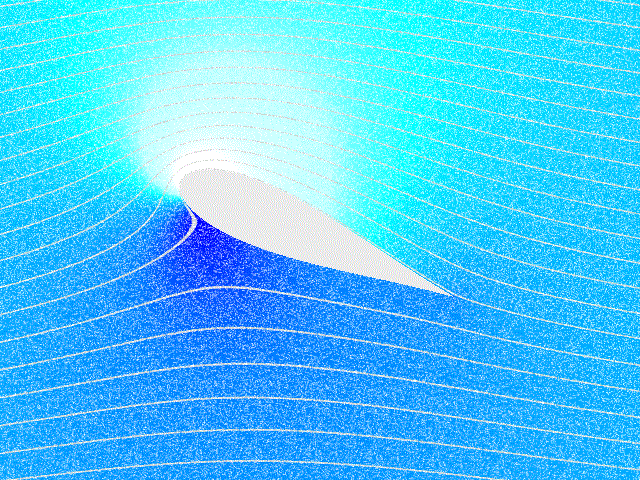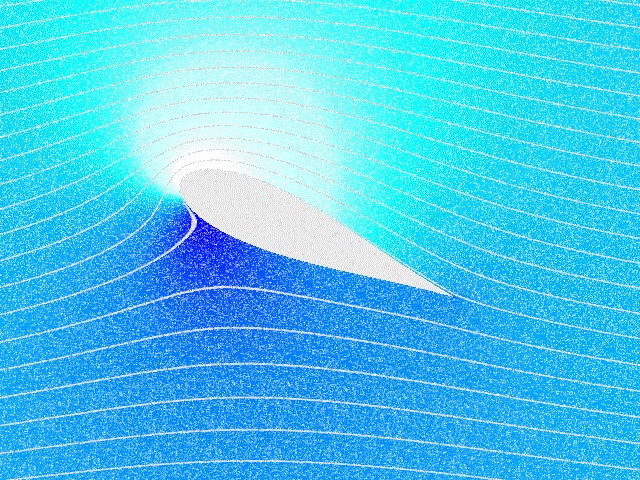
Fluid Dynamics
In physics, physical chemistry and engineering, fluid dynamics is a subdiscipline of fluid mechanics that describes the flow of fluids – liquids and gases. It has several subdisciplines, including (the study of air and other gases in motion) and (the study of water and other liquids in motion). Fluid dynamics has a wide range of applications, including calculating forces and moment (physics), moments on aircraft, determining the mass flow rate of petroleum through pipeline transport, pipelines, weather forecasting, predicting weather patterns, understanding nebulae in interstellar space, understanding large scale Geophysical fluid dynamics, geophysical flows involving oceans/atmosphere and Nuclear weapon design, modelling fission weapon detonation. Fluid dynamics offers a systematic structure—which underlies these practical disciplines—that embraces empirical and semi-empirical laws derived from flow measurement and used to solve practical problems. The solution to a fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |