|
IO-204
Hypoiodous acid is an inorganic compound with the chemical formula . It forms when an aqueous solution of iodine is treated with mercuric or silver salts. It rapidly decomposes by disproportionation: : Hypoiodous acid is a weak acid with a p''K''a of about 11. The conjugate base is hypoiodite (). Salts of this anion can be prepared by treating iodine with alkali hydroxides. They rapidly disproportionate to form iodides and iodates, but an iodine–hydroxide mixture can be used an in situ preparation of hypoiodite for other reactions. Ammonium hypoiodites can be formed by oxidation of the analogous iodide salts. These and also sodium hypoiodite are useful as oxidizing agents for a various types of organic compounds and also for a reaction analogous to the haloform reaction. Hypoiodite is one of the active oxidizing agents generated by lactoperoxidase as part of the mammalian innate immune system. Other oxyacids Hypoiodous acid is part of a series of oxyacids in which iodi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Dissociation Constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative property, quantitative measure of the acid strength, strength of an acid in Solution (chemistry), solution. It is the equilibrium constant for a chemical reaction :HA A^- + H^+ known as Dissociation (chemistry), dissociation in the context of acid–base reactions. The chemical species HA is an acid that dissociates into , called the conjugate base of the acid, and a hydron (chemistry), hydrogen ion, . The system is said to be in chemical equilibrium, equilibrium when the concentrations of its components do not change over time, because both forward and backward reactions are occurring at the same rate. The dissociation constant is defined by :K_\text = \mathrm, or by its logarithmic form :\mathrmK_\ce = - \log_ K_\text = \log_\frac where quantities in square brackets represent the molar concentrations of the species at equilibrium. For example ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Innate Immune System
The innate immune system or nonspecific immune system is one of the two main immunity strategies in vertebrates (the other being the adaptive immune system). The innate immune system is an alternate defense strategy and is the dominant immune system response found in plants, fungi, prokaryotes, and invertebrates (see #Beyond vertebrates, Beyond vertebrates).. The major functions of the innate immune system are to: * recruit immune cells to infection sites by producing chemical factors, including chemical mediators called cytokines * activate the complement cascade to identify bacteria, activate cells, and promote clearance of immune complex, antibody complexes or dead cells * identify and remove foreign substances present in organs, tissues, blood and lymph, by specialized white blood cells * activate the adaptive immune system through antigen presentation * act as a physical and chemical barrier to infectious agents; via physical measures such as skin and mucus, and chemical me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactoperoxidase
Lactoperoxidase (LPO, ) is a peroxidase enzyme secreted from mammary, salivary, tears and other mucosal glands including the lungs, bronchii and nose that function as a natural, first line of defense against bacteria and viral agents. Lactoperoxidase is a member of the animal heme-dependent peroxidases, heme peroxidase family of enzymes. In humans, lactoperoxidase is encoded by the ''LPO'' gene. Lactoperoxidase catalysis, catalyzes the oxidation of several inorganic and many organic enzyme substrate, substrates by hydrogen peroxide. Lactoperoxidase rapidly oxidizes iodide and slowly oxidizes bromide and is designated a haloperoxidase. Another important substrate is the pseudo-halide thiocyanate. The oxidized products display potent, non-specific bactericide, bactericidal and antiviral activities, including destruction of the influenza virus. Lactoperoxidase together with its inorganic ion substrates, hydrogen peroxide, DUOX1 and DUOX2 and products are termed the lactoperoxidase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
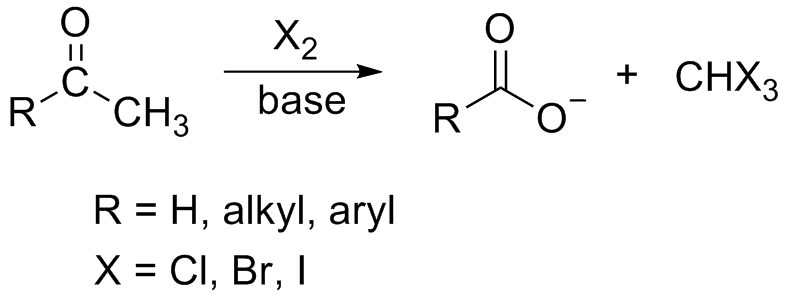
Haloform Reaction
In chemistry, the haloform reaction (also referred to as the Lieben haloform reaction) is a chemical reaction in which a haloform (, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group (, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups () or to produce chloroform (), bromoform (), or iodoform (). Note that fluoroform () can't be prepared in this way. Mechanism In the first step, the halogen dis-proportionates in the presence of hydroxide to give the halide and hypohalite. :Br2 + 2 OH- -> Br- + BrO- + H2O If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite: If a methyl ketone is present, it reacts with the hypohalite in a three-step process: 1. Under basic conditions, the ketone undergoes keto-enol tautomerisation. The enolate undergoes electrophilic attack by the hypohalite (containing a halo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing Agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hypoiodite
Sodium hypoiodite is an inorganic chemical used as an oxidant in various organic chemical reactions. It causes iodination of nitrogen atoms, such 1''H''-benzotriazole to give 1-iodo-1''H''-benzotriazole and an imine to give the analogous iodoimine. It oxidatively cleaves methyl ketones to give iodoform Iodoform (also known as triiodomethane) is the organoiodine compound with the chemical formula . It is a pale yellow, crystalline, volatile substance, with a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes refe .... References {{Sodium compounds Hypoiodites Sodium compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Hypoiodite
Ammonium hypoiodites are a class of reactive intermediates used in certain organic oxidation reactions. They consist of either ammonium itself or an alkylammonium with various substituents as cation, paired with a hypoiodite anion as the active oxidant. The hypoiodite is generated in situ from the analogous iodide reagent using peroxides, oxone, peracids, or other strong oxidizing agents. The hypoiodite is then capable of oxidizing various organic substrates. The iodide is regenerated, meaning the reaction runs with the iodide/hypoiodite as a catalyst in the presence of excess of the original strong oxidizing agent. Ammonium hypoiodites are capable of oxidizing benzylic methyl groups, initiating oxidative dearomatization, and oxidative decarboxylation of β-ketolactones. Similar to the β-ketolactone reaction, oxidative ether formation can be performed at the alpha position of various ketones. Using chiral ammonium cations can give high enantioselectivity of the alpha-etherificat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In Situ
is a Latin phrase meaning 'in place' or 'on site', derived from ' ('in') and ' ( ablative of ''situs'', ). The term typically refers to the examination or occurrence of a process within its original context, without relocation. The term is used across many disciplines to denote methods, observations, or interventions carried out in their natural or intended environment. By contrast, ' methods involve the removal or displacement of materials, specimens, or processes for study, preservation, or modification in a controlled setting, often at the cost of contextual integrity. The earliest known use of ''in situ'' in the English language dates back to the mid-17th century. In scientific literature, its usage increased from the late 19th century onward, initially in medicine and engineering. The natural sciences typically use methods to study phenomena in their original context. In geology, field analysis of soil composition and rock formations provides direct insights into Earth' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodate
An iodate is the polyatomic anion with the formula . It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. Iodate salts are often colorless. They are the salts of iodic acid. Structure Iodate is pyramidal in structure. The O–I–O angles range from 97° to 105°, somewhat smaller than the O–Cl–O angles in chlorate. Reactions Redox Iodate is one of several oxyanions of iodine, and has an oxidation number of +5. It participates in several redox reactions, such as the iodine clock reaction. Iodate shows no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate. Iodate is reduced by sulfite: : Iodate oxidizes iodide in acidic conditions: : Similarly, chlorate oxidizes iodide to iodate: : Iodate is also obtained by reducing a periodate with a sulfide. The byproduct of the reaction is a sulfoxide. Acid-base Iodate is unusual in that it forms a strong hydrogen bond with its parent ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is not. The l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |