|
AM-2233
AM-2233 is a drug that acts as a highly potent full agonist for the cannabinoid receptors, with a ''K''i of 1.8 nM at CB1 and 2.2 nM at CB2 as the active (''R'') enantiomer. It was developed as a selective radioligand for the cannabinoid receptors and has been used as its 131I derivative for mapping the distribution of the CB1 receptor in the brain. AM-2233 was found to fully substitute for THC in rats, with a potency lower than that of JWH-018 but higher than WIN 55,212-2. It is notable for inducing tinnitus, though the reasons for this are unclear and may provide valuable insight into tinnitus research. Legal Status As of October 2015 AM-2233 is a controlled substance in China. See also * AM-679 * AM-694 * AM-1220 * AM-1221 * AM-1235 * AM-1241 * AM-2232 * Cannabipiperidiethanone * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * List of HU cannabinoids * List of designer drugs Designer drugs are structural or functional anal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of AM Cannabinoids
Alexandros Makriyannis is a professor in the Department of Medicinal Chemistry at Northeastern University, where his research group has synthesized many new compounds with cannabinoid activity. Some of those are: See also * List of CP cannabinoids * List of JWH cannabinoids * List of HU cannabinoids A research group led by Raphael Mechoulam at Hebrew University has synthesized many cannabinoids. Some of those are: * HU-210 — a high affinity CB1 agonist (''K''i = 0.23 nM) * HU-211 — the (+)-enantiomer of HU-210 with dramatically re ... * List of miscellaneous designer cannabinoids References {{Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-1221
AM-1221 is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a ''K''i of 0.28 nM at CB2 and 52.3 nM at the CB1 receptor, giving it around 180 times selectivity for CB2. The 2- methyl and 6- nitro groups on the indole ring both tend to increase CB2 affinity while generally reducing affinity at CB1, explaining the high CB2 selectivity of AM-1221. However, despite this relatively high selectivity for CB2, its CB1 affinity is still too strong to make it useful as a truly selective CB2 agonist, so the related compound AM-1241 is generally preferred for research purposes. In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as AM-1221 are Schedule I Controlled Substances. Legal status It is illegal to supply, trade, sell, distribute, import or transport the pharmaceutical drug in the UK under the Psychoactive Substances Act 2016 which was inforce on May 26th 2016. See also * AM-630 * AM-1220 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
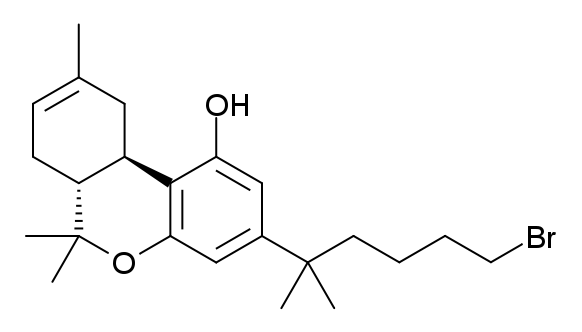
AM-2232
AM-2232 (1-(4-cyanobutyl)-3-(naphthalen-1-oyl)indole) is a drug that acts as a potent but unselective agonist for the cannabinoid receptors, with a ''K''i of 0.28 nM at CB1 and 1.48 nM at CB2. In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as AM-2232 are Schedule I Controlled Substances. See also * AM-694 * AM-1235 * AM-2233 * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids The John W. Huffman research group at Clemson University synthesized over 450 cannabinoids. Some of those are: [Baidu] |
AM-1220
AM-1220 is a drug that acts as a potent and moderately selective agonist for the cannabinoid receptor CB1, with around 19 times selectivity for CB1 over the related CB2 receptor. It was originally invented in the early 1990s by a team led by Thomas D'Ambra at Sterling Winthrop, but has subsequently been researched by many others, most notably the team led by Alexandros Makriyannis at the University of Connecticut. The (piperidin-2-yl)methyl side chain of AM-1220 contains a stereocenter, so there are two enantiomers with quite different potency, the (''R'')-enantiomer having a Ki of 0.27 nM at CB1 while the (''S'')-enantiomer has a much weaker Ki of 217 nM. Related compounds A number of related compounds are known with similar potent cannabinoid activity, with modifications such as substitution of the indole ring at the 2- or 6-positions, the naphthoyl ring substituted at the 4-position or replaced by substituted benzoyl rings or other groups, or the 1-(N-methylpiperi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-694
AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) is a designer drug that acts as a potent and selective agonist for the cannabinoid receptor CB1. It is used in scientific research for mapping the distribution of CB1 receptors. Pharmacology AM-694 is an agonist for cannabinoid receptors. It has a ''K''i of 0.08 nM at CB1 and 18 times selectivity over CB2 with a ''K''i of 1.44 nM. It is unclear what is responsible for this unusually high CB1 binding affinity, but it makes the 18F radiolabelled derivative of AM-694 useful for mapping the distribution of CB1 receptors in the body. Metabolism Pathways of metabolism include hydrolytic defluorination, carboxylation, and monohydroxylation of the ''N''-alkyl chain. See also * AM-679 * AM-1235 * AM-2201 * AM-2232 * AM-2233 * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids The John W. Huffman research group at Clemson University synthesized over 450 cannabinoids. Some of those are: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-679 (cannabinoid)
AM-679 (part of the AM cannabinoid series) is a drug that acts as a moderately potent agonist for the cannabinoid receptors, with a ''K''i of 13.5 nM at CB1 and 49.5 nM at CB2. AM-679 was one of the first 3-(2-iodobenzoyl)indole derivatives that was found to have significant cannabinoid receptor affinity, and while AM-679 itself has only modest affinity for these receptors, it was subsequently used as a base to develop several more specialised cannabinoid ligands that are now widely used in research, including the potent CB1 agonists AM-694 and AM-2233, and the selective CB2 agonist AM-1241. AM-679 was first identified as having been sold as a cannabinoid designer drug A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Des ... in Hungary in 2011, along with another novel c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabipiperidiethanone
Cannabipiperidiethanone (CPE or 1-(N-methylpiperidin-2-ylmethyl)-3-(2-methoxyphenylacetyl)indole) is a synthetic cannabinoid that has been found as an ingredient of "herbal" synthetic cannabis blends sold in Japan, alongside JWH-122 and JWH-081. Its binding affinity was measured at the CB1 and CB2 receptors and it was found to have an IC50 of 591 nM at CB1 and 968 nM at CB2, making it 2.3 times and 9.4 times weaker than JWH-250 at these two targets respectively. In the United States, CB1 receptor agonists of the 3-phenylacetylindole class such as cannabipiperidiethanone are Schedule I Controlled Substances. See also * AM-1220 * AM-1248 * AM-2233 * JWH-203 JWH-203 (1-pentyl-3-(2-chlorophenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist with approximately equal affinity at both the CB1 and CB2 receptors, having a Ki of 8.0 nM a ... * RCS-8 References External linksWater Soluble CBD Oil< ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FUBIMINA
FUBIMINA (also known as BIM-2201, BZ-2201 and FTHJ) is a synthetic cannabinoid that is the benzimidazole analog of AM-2201 and has been used as an active ingredient in synthetic cannabis products. It was first identified in Japan in 2013, alongside MEPIRAPIM. FUBIMINA acts as a reasonably potent agonist for the CB2 receptor ( ''K''i = 23.45 nM), with 12x selectivity over CB1 (''K''i = 296.1 nM), and does not fully substitute for Δ9-THC in rat discrimination studies. Related benzimidazole derivatives have been reported to be highly selective agonists for the CB2 receptor. See also * AM-694 * AM-1235 * AM-2232 * AM-2233 * BIM-018 * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * THJ-2201 THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 receptor and has been sold online as a designer drug. It is a structural analog of AM-2201 in which the central indole ring has been re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-1235
AM-1235 (1-(5-fluoropentyl)-3-(naphthalen-1-oyl)-6-nitroindole) is a drug that acts as a potent and reasonably selective agonist for the cannabinoid receptor CB1. Pharmacology Pharmacodynamics AM-1235 is a cannabinoid receptor agonist with Ki of 1.5 nM at CB1 compared to 20.4 nM at CB2. While the 6-nitro substitution on the indole ring reduces affinity for both CB1 and CB2 relative to the unsubstituted parent compound AM-2201, CB2 affinity is reduced much more, resulting in a CB1 selectivity of around 13 times. This is in contrast to other related compounds such as AM-1221 where a 6-nitro substitution instead confers significant selectivity for CB2. Pharmacokinetics AM-1235 metabolism differs only slightly from that of JWH-018. AM-1235 ''N''- dealkylation produces fluoropentane instead of pentane (or plain alkanes in general). It has been speculated that the fluoropentane might function as an alkylating agent or is further metabolized into toxic fluoroacetic aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-1241
AM-1241 (1-(methylpiperidin-2-ylmethyl)-3-(2-iodo-5-nitrobenzoyl)indole) is a chemical from the aminoalkylindole family that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 3.4 nM at CB2 and 80 times selectivity over the related CB1 receptor. It has analgesic effects in animal studies, particularly against "atypical" pain such as hyperalgesia and allodynia. This is thought to be mediated through CB2-mediated peripheral release of endogenous opioid peptides, as well as direct activation of the TRPA1 channel. It has also shown efficacy in the treatment of amyotrophic lateral sclerosis in animal models. Effects in bone cancer model The antihyperalgesic effects of AM-1241 were investigated in a murine bone cancer model. Sarcoma cells were injected into the femur of a mouse, and then mice were injected twice daily with AM-1241. Treatment with AM-1241 reduced both spontaneous and evoked pain, as well as reducing the bone loss and subseque ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Designer Drugs
Designer drugs are structural or functional analogues of controlled substances that are designed to mimic the pharmacological effects of the parent drug while avoiding detection or classification as illegal. Many of the older designer drugs (research chemicals) are structural analogues of psychoactive tryptamines or phenethylamines but there are many other chemically unrelated new psychoactive substances that can be considered part of the designer drug group. Designer drugs can also include substances that are not psychoactive in effect, such as analogues of controlled anabolic steroids and other performance and image enhancing drugs (PIEDs), including nootropics, weight loss drugs and erectile dysfunction medications. The pharmaceutical activities of these compounds might not be predictable based strictly upon structural examination. Many of the substances have common effects while structurally different or different effects while structurally similar due to SAR paradox. As a res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |