|
AM-1221
AM-1221 is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a ''K''i of 0.28 nM at CB2 and 52.3 nM at the CB1 receptor, giving it around 180 times selectivity for CB2. The 2- methyl and 6- nitro groups on the indole ring both tend to increase CB2 affinity while generally reducing affinity at CB1, explaining the high CB2 selectivity of AM-1221. However, despite this relatively high selectivity for CB2, its CB1 affinity is still too strong to make it useful as a truly selective CB2 agonist, so the related compound AM-1241 is generally preferred for research purposes. In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as AM-1221 are Schedule I Controlled Substances. Legal status It is illegal to supply, trade, sell, distribute, import or transport the pharmaceutical drug in the UK under the Psychoactive Substances Act 2016 which was inforce on May 26th 2016. See also * AM-630 * AM-1220 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM Cannabinoids
Alexandros Makriyannis is a professor in the Department of Medicinal Chemistry at Northeastern University, where his research group has synthesized many new compounds with cannabinoid activity. Some of those are: See also * List of CP cannabinoids * List of JWH cannabinoids * List of HU cannabinoids A research group led by Raphael Mechoulam at Hebrew University has synthesized many cannabinoids. Some of those are: * HU-210 — a high affinity CB1 agonist (''K''i = 0.23 nM) * HU-211 — the (+)-enantiomer of HU-210 with dramatically re ... * List of miscellaneous designer cannabinoids References {{Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-630
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a ''K''i of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist In pharmacology, partial agonists are drugs that bind to and activate a given receptor, but have only partial efficacy at the receptor relative to a full agonist. They may also be considered ligands which display both agonistic and antagonist .... It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives. See also * AM-1221 * Pravadoline * WIN 54,461 (6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-2233
AM-2233 is a drug that acts as a highly potent full agonist for the cannabinoid receptors, with a ''K''i of 1.8 nM at CB1 and 2.2 nM at CB2 as the active (''R'') enantiomer. It was developed as a selective radioligand for the cannabinoid receptors and has been used as its 131I derivative for mapping the distribution of the CB1 receptor in the brain. AM-2233 was found to fully substitute for THC in rats, with a potency lower than that of JWH-018 but higher than WIN 55,212-2. It is notable for inducing tinnitus, though the reasons for this are unclear and may provide valuable insight into tinnitus research. Legal Status As of October 2015 AM-2233 is a controlled substance in China. See also * AM-679 * AM-694 * AM-1220 * AM-1221 * AM-1235 * AM-1241 * AM-2232 * Cannabipiperidiethanone * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * List of HU cannabinoids * List of designer drugs Designer drugs are structural or functional anal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor 2
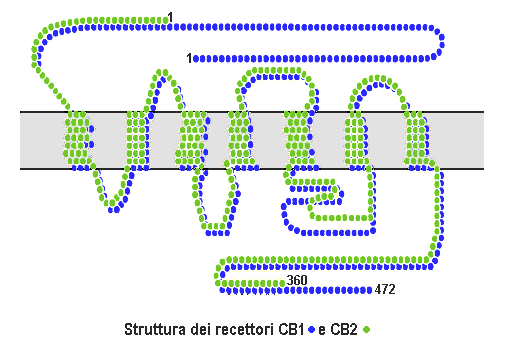
The cannabinoid receptor type 2, abbreviated as CB2, is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the ''CNR2'' gene. It is closely related to the cannabinoid receptor type 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids (plant cannabinoids). The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG). CB2 was cloned in 1993 by a research group from Cambridge looking for a second cannabinoid receptor that could explain the pharmacological properties of tetrahydrocannabinol. The receptor was identified among cDNAs based on its similarity in amino-acid sequence to the cannabinoid receptor type 1 (CB1) receptor, discovered in 1990. The discovery of this receptor helped provide a molecular explanation for the established effects of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor 1
Cannabinoid receptor type 1 (CB1), also known as cannabinoid receptor 1, is a G protein-coupled cannabinoid receptor that in humans is encoded by the ''CNR1'' gene. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by: endocannabinoids, a group of retrograde neurotransmitters that include anandamide and 2-arachidonoylglycerol (2-AG); plant phytocannabinoids, such as the compound THC which is an active ingredient of the psychoactive drug cannabis; and, synthetic analogs of THC. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin (THCV). The primary endogenous agonist of the human CB1 receptor is anandamide. Structure The CB1 receptor shares the structure characteristic of all G-protein-coupled receptors, possessing seven transmembrane domains connected by three extracellular and three intracellular loops, an extracellular N-terminal tail, and an intracellular C-terminal tail. The receptor ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MN-25
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a ''K''i of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a ''K''i of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a ''K''i of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098). Chemically, it is closely related to another indole-3-carboxamide synthetic cannabinoid, Org 28611, but with a different cycloalkyl substitution on the carboxamide, and the cyclohexylmethyl group replaced by morpholinylethyl, as in JWH-200 or A-796,260. Early compounds such as these have subsequently led to the development of many related indole-3-carboxamide cannabinoid ligands. See also * A-834,735 * AB-0 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UR-144
UR-144 (TMCP-018, KM-X1, MN-001, YX-17) is a drug invented by Abbott Laboratories, that acts as a selective full agonist of the peripheral cannabinoid receptor CB2, but with much lower affinity for the psychoactive CB1 receptor. Pharmacology UR-144 has high affinity for the CB2 receptor with a Ki of 1.8 nM but 83x lower affinity for the CB1 receptor with a Ki of 150 nM. UR-144 was found to possess an EC50 of 421 nM for human CB1 receptors, and 72 nM for human CB2 receptors. UR-144 produces bradycardia and hypothermia in rats at a dose of 10 mg/kg, suggesting weak cannabinoid-like activity. Chemically it is closely related to other 2,2,3,3-tetramethylcyclopropyl synthetic cannabinoids like A-796,260 and A-834,735 but with a different substitution on the 1-position of the indole core, in these compounds its 1-pentyl group is replaced with alkylheterocycles like 1-(2-morpholinoethyl) and 1-(tetrahydropyran-4-ylmethyl). Legality The UK ACMD recommended that generic prohibi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids; plant cannabinoids (such as Tetrahydrocannabinol, produced by the cannabis plant); and synthetic cannabinoids (such as HU-210). All of the endocannabinoids and phytocannabinoids (plant based cannabinoids) are lipophilic. There are two known subtypes of cannabinoid receptors, termed CB1 and CB2. The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system, in hematopoietic cells, and in parts of the brain. The protein sequences of CB1 and CB2 receptors are about 44% simila ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-1220
AM-1220 is a drug that acts as a potent and moderately selective agonist for the cannabinoid receptor CB1, with around 19 times selectivity for CB1 over the related CB2 receptor. It was originally invented in the early 1990s by a team led by Thomas D'Ambra at Sterling Winthrop, but has subsequently been researched by many others, most notably the team led by Alexandros Makriyannis at the University of Connecticut. The (piperidin-2-yl)methyl side chain of AM-1220 contains a stereocenter, so there are two enantiomers with quite different potency, the (''R'')-enantiomer having a Ki of 0.27 nM at CB1 while the (''S'')-enantiomer has a much weaker Ki of 217 nM. Related compounds A number of related compounds are known with similar potent cannabinoid activity, with modifications such as substitution of the indole ring at the 2- or 6-positions, the naphthoyl ring substituted at the 4-position or replaced by substituted benzoyl rings or other groups, or the 1-(N-methylpiperi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-1235
AM-1235 (1-(5-fluoropentyl)-3-(naphthalen-1-oyl)-6-nitroindole) is a drug that acts as a potent and reasonably selective agonist for the cannabinoid receptor CB1. Pharmacology Pharmacodynamics AM-1235 is a cannabinoid receptor agonist with Ki of 1.5 nM at CB1 compared to 20.4 nM at CB2. While the 6-nitro substitution on the indole ring reduces affinity for both CB1 and CB2 relative to the unsubstituted parent compound AM-2201, CB2 affinity is reduced much more, resulting in a CB1 selectivity of around 13 times. This is in contrast to other related compounds such as AM-1221 where a 6-nitro substitution instead confers significant selectivity for CB2. Pharmacokinetics AM-1235 metabolism differs only slightly from that of JWH-018. AM-1235 ''N''- dealkylation produces fluoropentane instead of pentane (or plain alkanes in general). It has been speculated that the fluoropentane might function as an alkylating agent or is further metabolized into toxic fluoroacetic aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Designer Drugs
A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Designer drugs include psychoactive substances that have been designated by the European Union as new psychoactive substances (NPS) as well as analogs of performance-enhancing drugs such as designer steroids. Some of these were originally synthesized by academic or industrial researchers in an effort to discover more potent derivatives with fewer side effects, and shorter duration (and possibly also because it is easier to apply for patents for new molecules) and were later co-opted for recreational use. Other designer drugs were prepared for the first time in clandestine laboratories. Because the efficacy and safety of these substances have not been thoroughly evaluated in animal and human trials, the use of some of these drugs may result i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidines
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name '' Piper'', which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson and again, independently, in 1852 by the French chemist Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst: : C5H5N + 3 H2 → C5H10NH Pyridine can also be re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |