Zirconocene Dichloride on:
[Wikipedia]
[Google]
[Amazon]
Zirconocene dichloride is an organozirconium compound composed of a

 The use of trimethylaluminium for this reaction results in exclusive formation of the ''syn''-addition product and, for terminal alkynes, the anti-Markovnikov addition with high selectivity (generally > 10:1). Unfortunately, the use of higher alkylaluminium reagents results in lowered yield, due to the formation of the hydroalumination product (via β-hydrogen elimination of the alkylzirconium intermediate) as side product, and only moderate regioselectivities. Thus, practical applications of the carboalumination reaction are generally confined to the case of methylalumination. Although this is a major limitation, the synthetic utility of this process remains significant, due to the frequent appearance of methyl-substituted alkenes in natural products.
The use of trimethylaluminium for this reaction results in exclusive formation of the ''syn''-addition product and, for terminal alkynes, the anti-Markovnikov addition with high selectivity (generally > 10:1). Unfortunately, the use of higher alkylaluminium reagents results in lowered yield, due to the formation of the hydroalumination product (via β-hydrogen elimination of the alkylzirconium intermediate) as side product, and only moderate regioselectivities. Thus, practical applications of the carboalumination reaction are generally confined to the case of methylalumination. Although this is a major limitation, the synthetic utility of this process remains significant, due to the frequent appearance of methyl-substituted alkenes in natural products.
zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
central atom, with two cyclopentadienyl Cyclopentadienyl can refer to
* Cyclopentadienyl anion, or cyclopentadienide,
** Cyclopentadienyl ligand
* Cyclopentadienyl radical, •
* Cyclopentadienyl cation,
See also
* Pentadienyl
{{Chemistry index ...
and two chloro ligands. It is a colourless diamagnetic
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagn ...
solid that is somewhat stable in air.
Preparation and structure
Zirconocene dichloride may be prepared from zirconium(IV) chloride-tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
complex and sodium cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pin ...
:
:ZrCl4(THF)2 + 2 NaCp → Cp2ZrCl2 + 2 NaCl + 2 THF
The closely related compound Cp2ZrBr2 was first described by Birmingham and Wilkinson.
The compound is a bent metallocene: the Cp rings are not parallel, the average Cp(centroid)-M-Cp angle being 128°. The Cl-Zr-Cl angle of 97.1° is wider than in niobocene dichloride (85.6°) and molybdocene dichloride (82°). This trend helped to establish the orientation of the HOMO
''Homo'' () is a genus of great ape (family Hominidae) that emerged from the genus ''Australopithecus'' and encompasses only a single extant species, ''Homo sapiens'' (modern humans), along with a number of extinct species (collectively called ...
in this class of complex.
Reactions
Schwartz's reagent
Zirconocene dichloride reacts withlithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula or . It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthe ...
to give Cp2ZrHCl Schwartz's reagent:
:(C5H5)2ZrCl2 + 1/4 LiAlH4 → (C5H5)2ZrHCl + 1/4 LiAlCl4
Since lithium aluminium hydride is a strong reductant, some over-reduction occurs to give the dihydrido complex, Cp2ZrH2; treatment of the product mixture with methylene chloride converts it to Schwartz's reagent.
Negishi reagent
Zirconocene dichloride can also be used to prepare the Negishi reagent, Cp2Zr( η2- butene), which can be used as a source of Cp2Zr in oxidative cyclisation reactions. The Negishi reagent is prepared by treating zirconocene dichloride with ''n''-BuLi, leading to replacement of the two chloride ligands with butyl groups. The dibutyl compound subsequently undergoes beta-hydride elimination to give one η2-butene ligand, with the other butyl ligand promptly lost asbutane
Butane () is an alkane with the formula C4H10. Butane exists as two isomers, ''n''-butane with connectivity and iso-butane with the formula . Both isomers are highly flammable, colorless, easily liquefied gases that quickly vaporize at ro ...
via reductive elimination.
Carboalumination
Zirconocene dichloride catalyzes the carboalumination of alkynes by trimethylaluminium to give a (alkenyl)dimethylalane, a versatile intermediate for further cross coupling reactions for the synthesis of stereodefined trisubstituted olefins. For example, α-farnesene can be prepared as a single stereoisomer by carboalumination of 1-buten-3-yne with trimethylaluminium, followed by palladium-catalyzed coupling of the resultant vinylaluminium reagent with geranyl chloride. The use of trimethylaluminium for this reaction results in exclusive formation of the ''syn''-addition product and, for terminal alkynes, the anti-Markovnikov addition with high selectivity (generally > 10:1). Unfortunately, the use of higher alkylaluminium reagents results in lowered yield, due to the formation of the hydroalumination product (via β-hydrogen elimination of the alkylzirconium intermediate) as side product, and only moderate regioselectivities. Thus, practical applications of the carboalumination reaction are generally confined to the case of methylalumination. Although this is a major limitation, the synthetic utility of this process remains significant, due to the frequent appearance of methyl-substituted alkenes in natural products.
The use of trimethylaluminium for this reaction results in exclusive formation of the ''syn''-addition product and, for terminal alkynes, the anti-Markovnikov addition with high selectivity (generally > 10:1). Unfortunately, the use of higher alkylaluminium reagents results in lowered yield, due to the formation of the hydroalumination product (via β-hydrogen elimination of the alkylzirconium intermediate) as side product, and only moderate regioselectivities. Thus, practical applications of the carboalumination reaction are generally confined to the case of methylalumination. Although this is a major limitation, the synthetic utility of this process remains significant, due to the frequent appearance of methyl-substituted alkenes in natural products.
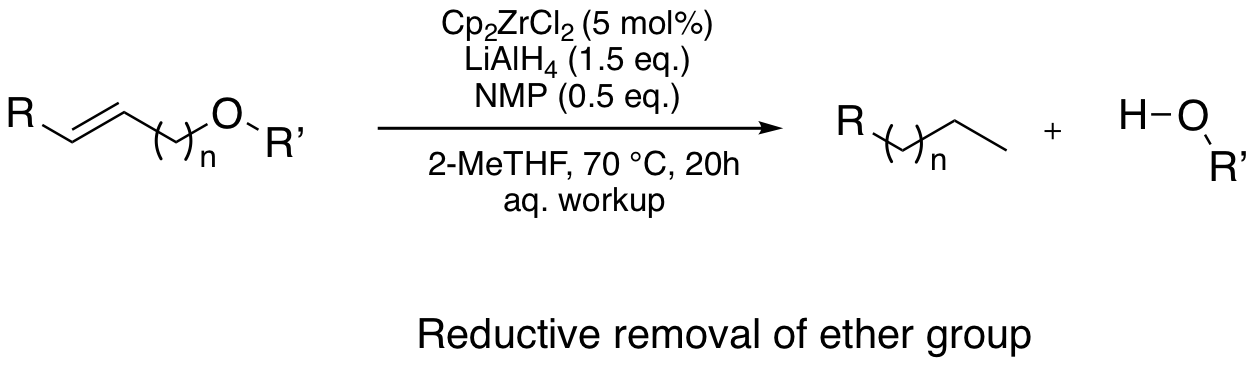
Zr-walk
Zirconocene dichloride together with a reducing reagent can form the zirconocene hydride catalyst in situ, which allows a positional isomerization (so-called "Zr-walk"), and ends up with a cleavage of allylic bonds. Not only individual steps under stoichiometric conditions were described with Schwartz reagent, and Negishi reagent, but also catalytic applications on alkene hydroaluminations, radical cyclisation, polybutadiene cleavage, and reductive removal of functional groups were reported.
References
Further reading
* {{DEFAULTSORT:Zirconocene Dichloride Organozirconium compounds Metallocenes Chloro complexes Cyclopentadienyl complexes Zirconium(IV) compounds