|
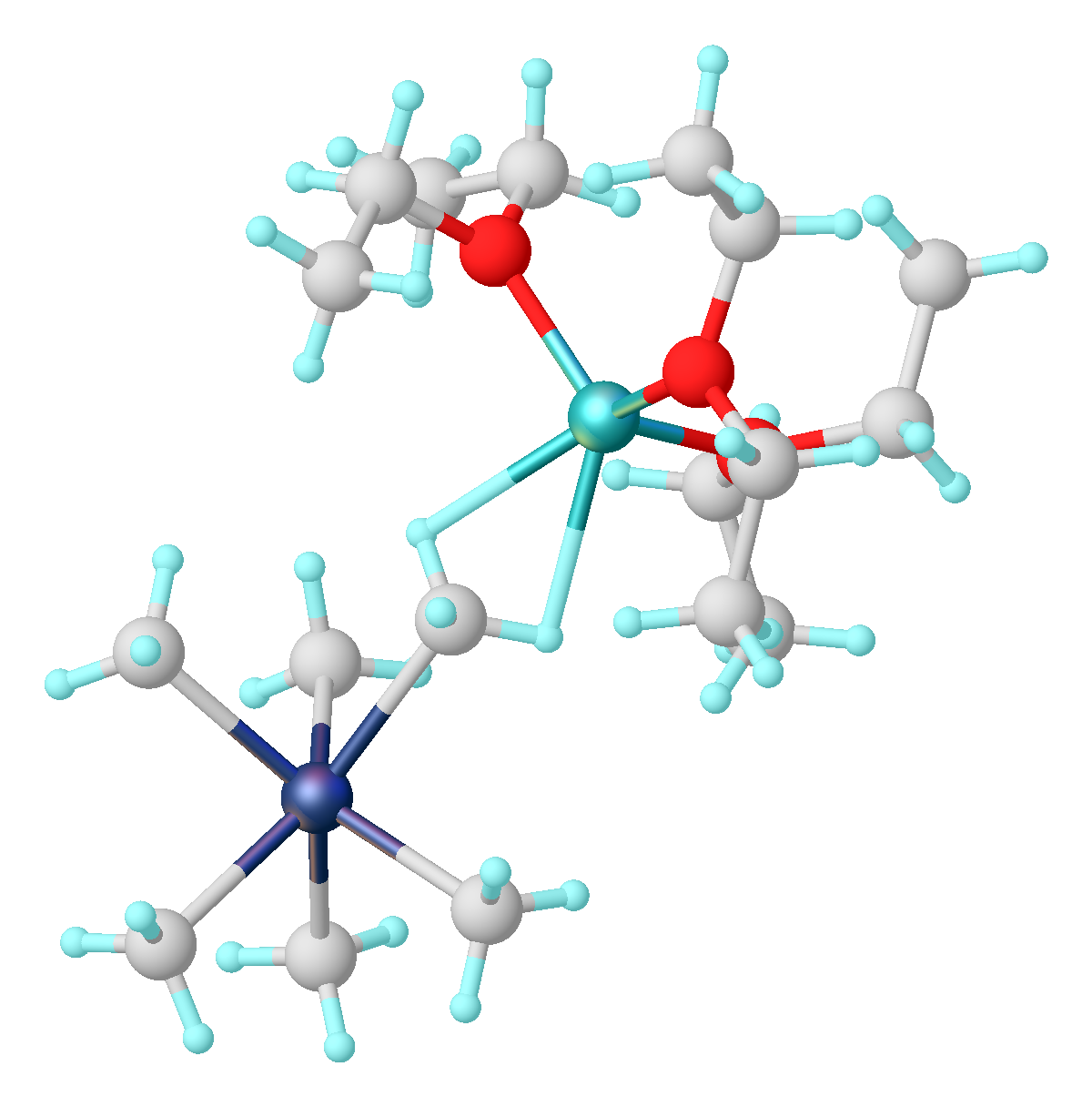
Niobocene Dichloride
Niobocene dichloride is the organometallic compound with the formula (C5H5)2NbCl2, abbreviated Cp2NbCl2. This paramagnetic brown solid is a starting reagent for the synthesis of other organoniobium compounds. The compound adopts a pseudotetrahedral structure with two cyclopentadienyl and two chloride substituents attached to the metal. A variety of similar compounds are known, including Cp2TiCl2. Preparation and structure It was originally reported by Geoffrey Wilkinson. It is prepared via a multistep reaction beginning with treatment of niobium pentachloride with cyclopentadienylsodium: : : : The compound adopts a "clamshell" structure characteristic of a bent metallocene where the Cp rings are not parallel, the average Cp(centroid)-M-Cp angle being about 130.3°. The Cl-Nb-Cl angle of 85.6° is narrower than in zirconocene dichloride (97.1°) but wider than in molybdocene dichloride (82°). This trend is consistent with the orientation of the HOMO in this class of complex. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorocarbon
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds that contain one or more carbon–chlorine bonds. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Organochlorides such as trichloroethylene, tetrachloroethylene, dichloromethane and chloroform are commonly used as solvents and are referred to as "chlorinated solvents". Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. They have higher ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bent Metallocene
In organometallic chemistry, bent metallocenes are a subset of metallocenes. In bent metallocenes, the ring systems coordinated to the metal are not parallel, but are tilted at an angle. A common example of a bent metallocene is Cp2TiCl2. Several reagents and much research is based on bent metallocenes. Synthesis Like regular metallocenes, bent metallocenes are synthesized by a variety of methods but most typically by reaction of sodium cyclopentadienide with the metal halide. This method applies to the synthesis of the bent metallocene dihalides of titanium, zirconium, hafnium, and vanadium: :2 NaC5H5 + TiCl4 → (C5H5)2TiCl2 + 2 NaCl In the earliest work in this area, Grignard reagents were used to deprotonate the cyclopentadiene. Niobocene dichloride, featuring Nb(IV), is prepared via a multistep reaction that begins with a Nb(V) precursor: :NbCl5 + 6 NaC5H5 → 5 NaCl + (C5H5)4Nb + organic products :(C5H5)4Nb + 2 HCl + 0.5 O2) → 2O">sub>2Ol2 + 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienyl Complexes
Cyclopentadienyl can refer to *Cyclopentadienyl anion Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pin ..., or cyclopentadienide, ** Cyclopentadienyl ligand * Cyclopentadienyl radical, • * Cyclopentadienyl cation, See also * Pentadienyl {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoniobium Compounds
Organoniobium chemistry is the chemistry of compounds containing niobium-carbon (Nb-C) bonds. Compared to the other group 5 transition metal Organometallic chemistry, organometallics, the chemistry of organoniobium compounds most closely resembles that of Organotantalum chemistry, organotantalum compounds. Organoniobium compounds of oxidation states +5, +4, +3, +2, +1, 0, −1, and −3 have been prepared, with the +5 oxidation state being the most common. Compound classes Carbonyls Unlike vanadium, which forms the neutral hexacarbonyl, niobium does not easily form an analogous complex. The salts of the anionic binary carbonyl, , are however well characterized. They are obtained by reduction of under an atmosphere of CO. Alkyl A wide variety of alkyl Nb compounds have been prepared. Low coordination number complexes require the absence of any β-hydrogen to prevent rapid β-hydride elimination. The simplest compounds are salts of , which is prepared by alkylation of using m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallocenes
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metallic element, metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride or vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalysis, catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze Ziegler–Natta catalyst, olefin polymerization. Some metallocenes consist of metal plus two cyclooctatetraenide anions (, abbreviated cot2−), namely the lanthanocenes and the actinocenes (uranocene and others). Metallocenes are a subset of a broader class of compounds called sandwich compounds. In the structure shown at right, the two pentagons are the cyclopentadienyl anions with circles inside them indicating they are aromaticity, aromatically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanocene Dichloride
Titanocene dichloride is the organotitanium compound with the formula (hapticity, ''η''5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug. Preparation and structure The standard preparations of Cp2TiCl2 start with titanium tetrachloride. The original synthesis by Geoffrey Wilkinson, Wilkinson and Birmingham, using sodium cyclopentadienide, is still commonly used: :2 NaC5H5 + TiCl4 → (C5H5)2TiCl2 + 2 NaCl It can also be prepared by using freshly distilled cyclopentadiene rather than its sodium derivative: :2 C5H6 + TiCl4 → (C5H5)2TiCl2 + 2 HCl Focusing on the geometry of the Ti center, Cp2TiCl2 adopts a distorted tetrahedral geometry (counting Cp as a monodentate ligand). The Ti-Cl distance is 2.37 Å ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HOMO/LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontier orbitals'', such as in the frontier molecular orbital theory. Gap The energy difference between the HOMO and LUMO is ''the HOMO–LUMO gap''. Its size can be used to predict the strength and stability of transition metal complexes, as well as the colors they produce in solution. As a rule of thumb, the smaller a compound's HOMO–LUMO gap, the less stable the compound. Recent quantum‐chemical analyses of over 700 compounds demonstrated that terrestrial secondary metabolites exhibit HOMO–LUMO gaps on average about 2 eV narrower than organic molecules found in carbonaceous meteorites, and that combining gap width with hydrophilicity creates a robust discriminator between biotic and abiotic chemistries. This suggests that the HOMO–LU ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdocene Dichloride
Molybdocene dichloride is the organomolybdenum compound with the formula ( η5-C5H5)2MoCl2 and IUPAC The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ... name dichlorobis(η5-cyclopentadienyl)molybdenum(IV), and is commonly abbreviated as Cp2MoCl2. It is a brownish-green air- and moisture-sensitive powder. In the research laboratory, it is used to prepare many derivatives. Preparation and structure The compound is prepared from molybdocene dihydride by treatment with chloroform: :(C5H5)2MoH2 + 2 CHCl3 → (C5H5)2MoCl2 + 2 CH2Cl2 The compound adopts a "clamshell" structure where the Cp rings are not parallel, the average Cp(centroid)-M-Cp angle being 130.6°. The two chloride ligands are cis, the Cl-Mo-Cl angle of 82° being narrower than in niobocene dichloride (85.6°), which in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconocene Dichloride
Zirconocene dichloride is an organozirconium compound composed of a zirconium central atom, with two cyclopentadienyl and two chloro ligands. It is a colourless diamagnetic solid that is somewhat stable in air. Preparation and structure Zirconocene dichloride may be prepared from zirconium(IV) chloride-tetrahydrofuran complex and sodium cyclopentadienide: :ZrCl4(THF)2 + 2 NaCp → Cp2ZrCl2 + 2 NaCl + 2 THF The closely related compound Cp2ZrBr2 was first described by Birmingham and Wilkinson. The compound is a bent metallocene: the Cp rings are not parallel, the average Cp(centroid)-M-Cp angle being 128°. The Cl-Zr-Cl angle of 97.1° is wider than in niobocene dichloride (85.6°) and molybdocene dichloride (82°). This trend helped to establish the orientation of the HOMO in this class of complex. Reactions Schwartz's reagent Zirconocene dichloride reacts with lithium aluminium hydride to give Cp2ZrHCl Schwartz's reagent: :(C5H5)2ZrCl2 + 1/4 LiAlH4 → (C5H5)2ZrHCl + 1/4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanocene Dichloride
Titanocene dichloride is the organotitanium compound with the formula (hapticity, ''η''5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug. Preparation and structure The standard preparations of Cp2TiCl2 start with titanium tetrachloride. The original synthesis by Geoffrey Wilkinson, Wilkinson and Birmingham, using sodium cyclopentadienide, is still commonly used: :2 NaC5H5 + TiCl4 → (C5H5)2TiCl2 + 2 NaCl It can also be prepared by using freshly distilled cyclopentadiene rather than its sodium derivative: :2 C5H6 + TiCl4 → (C5H5)2TiCl2 + 2 HCl Focusing on the geometry of the Ti center, Cp2TiCl2 adopts a distorted tetrahedral geometry (counting Cp as a monodentate ligand). The Ti-Cl distance is 2.37 Å ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niobium Pentachloride
Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium. NbCl5 may be purified by sublimation. Structure and properties Niobium(V) chloride forms chloro-bridged dimers in the solid state (''see'' figure). Each niobium centre is six-coordinate, but the octahedral coordination is significantly distorted. The equatorial niobium–chlorine bond lengths are 225 pm (terminal) and 256 pm (bridging), whilst the axial niobium-chlorine bonds are 229.2 pm and are deflected inwards to form an angle of 83.7° with the equatorial plane of the molecule. The Nb–Cl–Nb angle at the bridge is 101.3°. The Nb–Nb distance is 398.8 pm, too long for any metal-metal interaction. NbBr5, NbI5, TaCl5 TaBr5 and TaI5 are isostructural with NbCl5. Preparation Industrially, niobium pentachloride is obtai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |