Yellow Phosphorus on:
[Wikipedia]
[Google]
[Amazon]
White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is an allotrope of phosphorus. It is a translucent
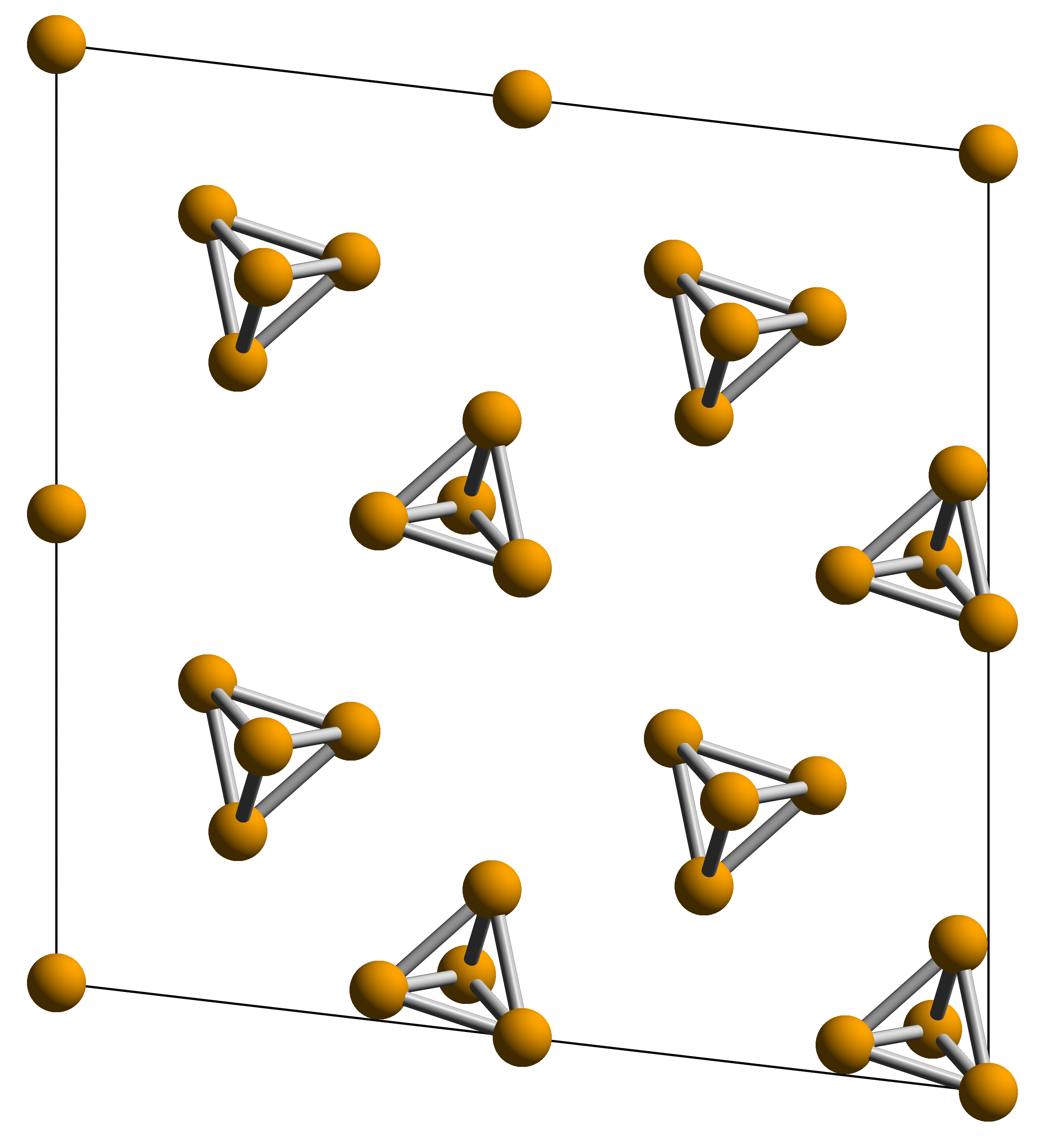 White phosphorus exists as
White phosphorus exists as
wax
Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give lo ...
y solid that quickly yellows in light (due to its photochemical
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible (400–750&nb ...
conversion into red phosphorus
Red phosphorus is an Allotropes of phosphorus, allotrope of phosphorus. It is an amorphous polymeric red solid that is stable in air. It can be easily converted from white phosphorus under light or heating. It finds applications as matches and fir ...
), and impure white phosphorus is for this reason called yellow phosphorus. White phosphorus is the first allotrope of phosphorus, and in fact the first elementary substance
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
to be discovered that was not known since ancient times. It glows greenish in the dark (when exposed to oxygen) and is highly flammable
A combustible material is a material that can burn (i.e., sustain a flame) in air under certain conditions. A material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort ...
and pyrophoric
A substance is pyrophoric (from , , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylb ...
(self-igniting) upon contact with air. It is toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
, causing severe liver damage
Liver disease, or hepatic disease, is any of many diseases of the liver. If long-lasting it is termed chronic liver disease. Although the diseases differ in detail, liver diseases often have features in common.
Liver diseases
File:Ground glas ...
on ingestion and phossy jaw
Phossy jaw, formally known as phosphorus necrosis of the jaw, was an occupational disease affecting those who worked with white phosphorus (also known as ''yellow phosphorus'') without proper safeguards. It is also likely to occur as the result ...
from chronic ingestion or inhalation. The odour of combustion of this form has a characteristic garlic odor, and samples are commonly coated with white "diphosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4 O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrat ...
", which consists of tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and can be stored under water. is soluble in benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, oils
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) and lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturat ...
, carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
, and disulfur dichloride
Disulfur dichloride (or disulphur dichloride by the British English spelling) is the inorganic compound of sulfur and chlorine with the Chemical formula, formula . It is an amber oily liquid.
Sometimes, this compound is incorrectly named ''sulfur ...
.
Structure
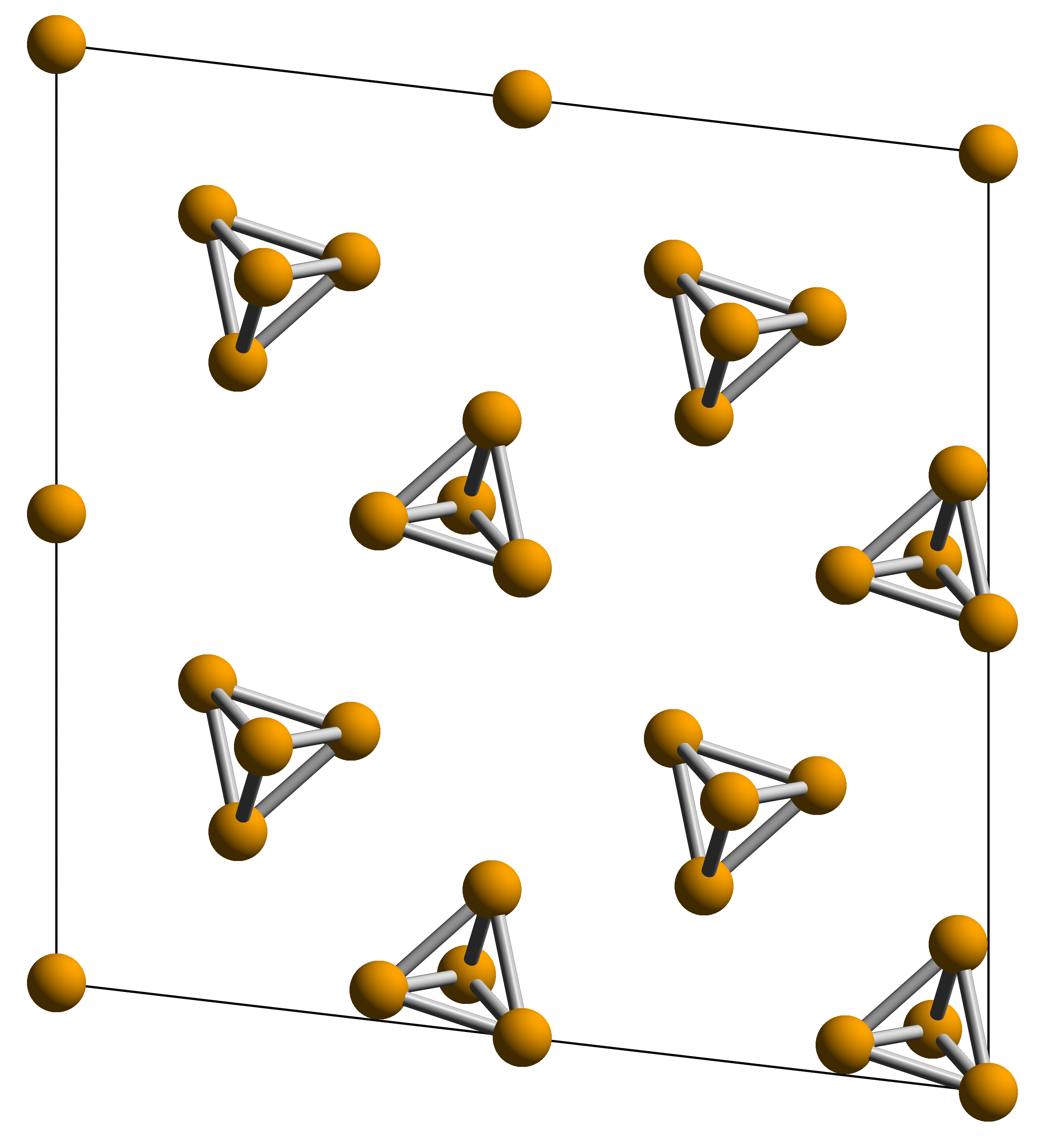 White phosphorus exists as
White phosphorus exists as molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
of four phosphorus atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
in a tetrahedral structure, joined by six phosphorus—phosphorus single bonds. The tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
arrangement results in ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles ar ...
and instability. Although both are called "white phosphorus", in fact two different crystal allotropes are known, interchanging reversibly at 195.2 K. The element's standard state
The standard state of a material (pure substance, mixture or solution) is a reference point used to calculate its properties under different conditions. A degree sign (°) or a superscript ⦵ symbol (⦵) is used to designate a thermodynamic q ...
is the body-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal structure#Unit cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There ...
α form, which is actually metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
under standard conditions
Standard temperature and pressure (STP) or standard conditions for temperature and pressure are various standard sets of conditions for experimental measurements used to allow comparisons to be made between different sets of data. The most used ...
. The β form is believed to have a hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is d ...
crystal structure.
Molten and gaseous white phosphorus also retains the tetrahedral molecules, until when it starts decomposing to molecules. The molecule in the gas phase has a P-P bond length of ''r''g = 2.1994(3) Å as was determined by gas electron diffraction
Gas electron diffraction (GED) is one of the applications of electron diffraction techniques. The target of this method is the determination of the structure of gaseous molecules, i.e., the geometrical arrangement of the atoms from which a molec ...
. The β form of white phosphorus contains three slightly different molecules, i.e. 18 different P-P bond lengths — between 2.1768(5) and 2.1920(5) Å. The average P-P bond length is 2.183(5) Å.
Chemical properties
Despite white phosphorus not being the most stable allotropes of phosphorus, its molecular nature allows it to be easily purified. Thus, it is defined to have a zeroenthalpy of formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, wi ...
.
In base, white phosphorus spontaneously disproportionates
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionatio ...
to phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
and various phosphorus oxyacid
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce ...
salts.
Many reactions of white phosphorus involve insertion into the P-P bonds, such as the reaction with oxygen, sulfur, phosphorus tribromide
Phosphorus tribromide is a colourless liquid with the formula P Br3. The liquid fumes in moist air due to hydrolysis and has a penetrating odour. It is used in the laboratory for the conversion of alcohols to alkyl bromides.
Preparation
PBr3 ...
and the NO+ ion.
It ignites spontaneously in air at about , and at much lower temperatures if finely divided (due to melting-point depression
:''This article deals with melting/freezing point depression due to very small particle size. For depression due to the mixture of another compound, see freezing-point depression.''
Melting-point depression is the phenomenon of reduction of the m ...
). Phosphorus reacts with oxygen, usually forming ''two'' oxides depending on the amount of available oxygen: (phosphorus trioxide
Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, a ...
) when reacted with a limited supply of oxygen, and when reacted with excess oxygen. On rare occasions, , , and are also formed, but in small amounts. This combustion gives phosphorus(V) oxide:
:
Production and applications
The white allotrope can be produced using several methods. In the industrial process,phosphate rock
Phosphorite, phosphate rock or rock phosphate is a non- detrital sedimentary rock that contains high amounts of phosphate minerals. The phosphate content of phosphorite (or grade of phosphate rock) varies greatly, from 4% to 20% phosphorus pentox ...
is heated in an electric or fuel-fired furnace in the presence of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
and silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
.Threlfall, R.E., (1951). ''100 years of Phosphorus Making: 1851–1951''. Oldbury: Albright and Wilson Albright may refer to:
*Albright (surname)
*Albright, Alberta, Canada
*Albright, West Virginia, United States
*Albright College, a liberal arts college located in Reading, Pennsylvania, United States
*Albright–Knox Art Gallery, Buffalo, New York, ...
Ltd Elemental phosphorus is then liberated as a vapour and can be collected under phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...
. An idealized equation for this carbothermal reaction is shown for calcium phosphate
The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. Calcium phosphates are white ...
(although phosphate rock contains substantial amounts of fluoroapatite, which would also form silicon tetrafluoride
Silicon tetrafluoride or tetrafluorosilane is a chemical compound with the formula Si F4. This colorless gas is notable for having a narrow liquid range: its boiling point is only 4 °C above its melting point. It was first prepared in 1771 ...
):
:
In this way, an estimated 750,000 tons were produced in 1988.
Most (83% in 1988) white phosphorus is used as a precursor to phosphoric acid, half of which is used for food or medical products where purity is important. The other half is used for detergents. Much of the remaining 17% is mainly used for the production of chlorinated compounds phosphorus trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic ...
, phosphorus oxychloride
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula . It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phos ...
, and phosphorus pentachloride
Phosphorus pentachloride is the chemical compound with the formula . It is one of the most important phosphorus chlorides/oxychlorides, others being and . finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, althoug ...
:
:
Other products derived from white phosphorus include phosphorus pentasulfide
Phosphorus pentasulfide is the inorganic compound with the formula (empirical) or ( molecular). This yellow solid is the one of two phosphorus sulfides of commercial value. Samples often appear greenish-gray due to impurities. It is soluble in ...
and various metal phosphides.
Other polyhedrane analogues
Although white phosphorus forms thetetrahedron
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
, the simplest possible Platonic solid
In geometry, a Platonic solid is a Convex polytope, convex, regular polyhedron in three-dimensional space, three-dimensional Euclidean space. Being a regular polyhedron means that the face (geometry), faces are congruence (geometry), congruent (id ...
, no other polyhedral phosphorus clusters are known. White phosphorus converts to the thermodynamically-stabler red allotrope, but that allotrope is not isolated polyhedra.
A cubane-type cluster
A cubane-type cluster is an arrangement of atoms in a molecular structure that forms a cube. In the simplest case, the eight vertices are symmetry equivalent and the species has Oh symmetry group, symmetry. Such structure occurs in the hydrocarbon ...
, in particular, is unlikely to form, and the closest approach is the half-phosphorus compound , produced from phosphaalkyne
In chemistry, a phosphaalkyne (International Union of Pure and Applied Chemistry, IUPAC name: alkylidynephosphane) is an organophosphorus compound containing a triple bond between phosphorus and carbon with the general chemical formula . Phosphaa ...
s. Other clusters are more thermodynamically favorable, and some have been partially formed as components of larger polyelemental compounds.
Safety
White phosphorus is acutely toxic, with a lethal dose of 50-100 mg (1 mg/kg body weight). Its mode of action is not known but is thought to involve its reducing properties, possibly forming intermediate reducing compounds such as hypophosphite, phosphite, and phosphine. It damages the liver, kidneys, and other organs before eventually being metabolized to non-toxic phosphate. Chronic low-level exposure leads to tooth loss andphossy jaw
Phossy jaw, formally known as phosphorus necrosis of the jaw, was an occupational disease affecting those who worked with white phosphorus (also known as ''yellow phosphorus'') without proper safeguards. It is also likely to occur as the result ...
which appears to be caused by the formation of amino bisphosphonates.
White phosphorus is used as a weapon because it is pyrophoric. For the same reasons, it is dangerous to handle. Measures are taken to protect samples from air since it will react with oxygen at ambient temperatures, and even in small samples this can lead to self-heating and eventual combustion. There are anecdotal reports of problems for beachcombers
''The Beachcombers'' is a Canadian comedy drama television series that ran on CBC Television from October 1, 1972, to December 12, 1990. With over 350 episodes, it is one of the longest-running dramatic series ever made for Canadian English-langu ...
who may collect washed-up samples while unaware of their true nature.
See also
*Red phosphorus
Red phosphorus is an Allotropes of phosphorus, allotrope of phosphorus. It is an amorphous polymeric red solid that is stable in air. It can be easily converted from white phosphorus under light or heating. It finds applications as matches and fir ...
* Allotropes of phosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
White phosphorus
...
* Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
References
{{reflist Allotropes