Winstein Reaction on:
[Wikipedia]
[Google]
[Amazon]
 In
In

 There has been some debate over how much the pi-bonded resonance structure actually contributes to the delocalized electronic structure. Through H and C
There has been some debate over how much the pi-bonded resonance structure actually contributes to the delocalized electronic structure. Through H and C
 According to proponents of a classical double-well potential, the 2-norbornyl cation exists in
According to proponents of a classical double-well potential, the 2-norbornyl cation exists in


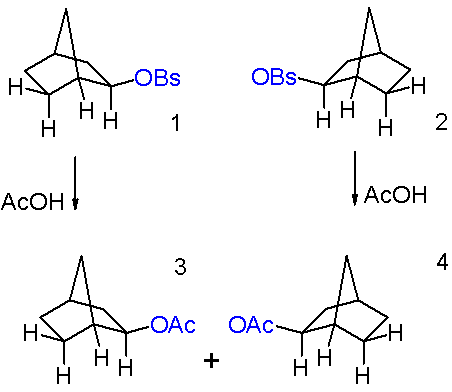 When a single
When a single

 Radioactive isotope labeling experiments provide a powerful tool for determining the structure of organic molecules. By systematically decomposing the 2-norbornyl cation and analyzing the amount of radioactive isotope in each decomposition product, researchers were able to show further evidence for the non-classical picture of delocalized bonding (''see Figure 9''). Proponents of the nonclassical picture would expect 50% of the generated CO2 in the decomposition in Figure 9 to contain 14C, while proponents of the classical picture would expect more of the generated CO2 to be radioactive due to the short-lived nature of the cation. 40% of the carbon dioxide produced via decomposition has been observed to be radioactive, suggesting that the non-classical picture is more correct.
Further distinction between non-classical and classical structures of the 2-norbornyl cation is possible by combining NMR experiments with isotope-labeling experiments. Isotopic substitution of one of two deuterium atoms for a hydrogen atom causes the environment of nearby NMR-active atoms to change dramatically. Asymmetric deuterium isotope labeling (substitution) will cause a set of carbons that were all equivalent in the all-hydrogen species to be split into two or more sets of equivalent carbons in the deutero-labeled species; this will be manifested in the NMR spectrum as one peak in the all-hydrogen species' spectrum becoming at least two "split" peaks in the deutero-labeled species. If a system is undergoing a rapid equilibrium at a rate faster than the timescale of a C NMR experiment, the relevant peak will be split dramatically (on the order of 10-100 ppm). If the system is instead static, the peak will be split very little. The C NMR spectrum of the 2-norbornyl cation at -150 °C shows that the peaks corresponding to carbons 1 and 2 are split by less than 10 ppm (parts per million) when this experiment is carried out, indicating that the system is not undergoing a rapid equilibrium as in the classical picture.
Radioactive isotope labeling experiments provide a powerful tool for determining the structure of organic molecules. By systematically decomposing the 2-norbornyl cation and analyzing the amount of radioactive isotope in each decomposition product, researchers were able to show further evidence for the non-classical picture of delocalized bonding (''see Figure 9''). Proponents of the nonclassical picture would expect 50% of the generated CO2 in the decomposition in Figure 9 to contain 14C, while proponents of the classical picture would expect more of the generated CO2 to be radioactive due to the short-lived nature of the cation. 40% of the carbon dioxide produced via decomposition has been observed to be radioactive, suggesting that the non-classical picture is more correct.
Further distinction between non-classical and classical structures of the 2-norbornyl cation is possible by combining NMR experiments with isotope-labeling experiments. Isotopic substitution of one of two deuterium atoms for a hydrogen atom causes the environment of nearby NMR-active atoms to change dramatically. Asymmetric deuterium isotope labeling (substitution) will cause a set of carbons that were all equivalent in the all-hydrogen species to be split into two or more sets of equivalent carbons in the deutero-labeled species; this will be manifested in the NMR spectrum as one peak in the all-hydrogen species' spectrum becoming at least two "split" peaks in the deutero-labeled species. If a system is undergoing a rapid equilibrium at a rate faster than the timescale of a C NMR experiment, the relevant peak will be split dramatically (on the order of 10-100 ppm). If the system is instead static, the peak will be split very little. The C NMR spectrum of the 2-norbornyl cation at -150 °C shows that the peaks corresponding to carbons 1 and 2 are split by less than 10 ppm (parts per million) when this experiment is carried out, indicating that the system is not undergoing a rapid equilibrium as in the classical picture.
Nobel prize non-classical ion
{{DEFAULTSORT:Norbornyl cation, 2- Carbocations Physical organic chemistry Reactive intermediates
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the term 2-norbornyl cation (or 2-bicyclo .2.1eptyl cation) describes a carbonium ion
In chemistry, a carbonium ion is a cation that has a pentacoordinated carbon atom. They are a type of carbocation. In older literature, the name "carbonium ion" was used for what is today called carbenium. Carbonium ions charge is delocalized ...
ic derivative of norbornane
Norbornane (also known as bicyclo .2.1eptane) is an organic compound and a saturated hydrocarbon with chemical formula C7H12. It is a crystalline compound with a melting point of 88 °C. The carbon skeleton is derived from cyclohexane ring with ...
. A salt of the 2-norbornyl cation was crystallized and characterized by X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
confirmed the non-classical structure.
Theory

Hypovalency: the non-classical picture
Advocates of the non-classical nature of the stable 2-norbornyl cation typically depict the species using eitherresonance structures
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
or a single structure with partial bonds (''see Figure 2''). This hypovalent interaction can be imagined as the net effect of i) a partial sigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
between carbons 1 and 6, ii) a partial sigma bond between carbons 2 and 6, and iii) a partial pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
between carbons 1 and 2. Each partial bond is represented as a full bond in one of the three resonance structures or as a dashed partial bond if the cation is depicted through a single structure.
 There has been some debate over how much the pi-bonded resonance structure actually contributes to the delocalized electronic structure. Through H and C
There has been some debate over how much the pi-bonded resonance structure actually contributes to the delocalized electronic structure. Through H and C NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic f ...
, it has been confirmed that significant positive charge lies on methylene carbon 6. This is surprising as primary carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
s are much less stable than secondary carbocations. However, the 2-norbornyl cation can be formed from derivatives of β-(Δ-cyclopentenyl)-ethane, indicating that the pi-bonded resonance structure is significant.
The 2-norbornyl cation was one of the first examples of a ''non-classical ion''. Non-classical ions can be defined as organic cations in which electron density of a filled bonding orbital
In theoretical chemistry, the bonding orbital is used in molecular orbital (MO) theory to describe the attractive interactions between the atomic orbitals of two or more atoms in a molecule. In MO theory, electrons are portrayed to move in waves. ...
is shared over three or more centers and contains some sigma-bond character. The 2-norbornyl cation is seen as the prototype for non-classical ions. Other simple cations such as protonated acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
(ethynium, ), protonated ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
(ethenium, ), and protonated ethane
Ethane ( , ) is a naturally occurring Organic compound, organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is List of purification methods ...
(ethanium, ) have been shown to be best described as non-classical through infrared spectroscopy.
The most frequently proposed molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
depiction of the 2-norbornyl cation is shown in Figure 3. Two ''p''-type orbitals, one on each of carbons 1 and 2, interact with a ''sp''-hybridized orbital on carbon 6 to form the hypovalent bond. Extended Hückel Theory calculations for the 2-norbornyl cation suggest that the orbital on carbon 6 could instead be ''sp''-hybridized, though this only affects the geometry of the geminal
In chemistry, the descriptor geminal () refers to the relationship between two atoms or functional groups that are attached to the same atom. A geminal diol, for example, is a diol (a molecule that has two alcohol functional groups) attached to ...
hydrogens.
Rapid equilibrium: the classical picture
 According to proponents of a classical double-well potential, the 2-norbornyl cation exists in
According to proponents of a classical double-well potential, the 2-norbornyl cation exists in dynamic equilibrium
In chemistry, a dynamic equilibrium exists once a reversible reaction occurs. Substances initially transition between the reactants and products at different rates until the forward and backward reaction rates eventually equalize, meaning the ...
between two enantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...
ic asymmetric structures. The delocalized species central to the non-classical picture is merely a transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
between the two structures. Wagner-Meerwein rearrangements are invoked as the mechanism that converts between the two enantiomers (''see Figure 4'').
Efforts to isolate the asymmetric species spectroscopically are typically unsuccessful. The major reason for this failure is reported to be extremely rapid forward and reverse reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
s, which indicate a very low potential barrier for interconversion between the two enantiomers.
Nortricyclonium: another non-classical structure
Some chemists have also considered the 2-norbornyl cation to be best represented by the nortricyclonium ion, a C-symmetric protonated nortricyclene. This depiction was first invoked to partially explain results of a Cisotope scrambling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through chemical reaction, metabolic pathway, or a Cell (biology), biological cell. The reactant ...
experiment. The molecular orbital representation of this structure involves an in-phase interaction between ''sp''-hybridized orbitals from carbons 1, 2 and 6 and the 1s atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
on a shared hydrogen atom (''see Figure 5'').

History
Non-classical ions
Non-classical ions differ from traditional cations in theirelectronic structure
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
: though chemical bonds are typically depicted as the sharing of electrons between two atoms, stable non-classical ions can contain three or more atoms that share a single pair of electrons. In 1939, Thomas Nevell and others attempted to elucidate the mechanism for transforming camphene
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As with other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell. It is a minor constituent of many essential oils such as tu ...
hydrochloride into isobornyl
Borneol is a bicyclic organic compound and a terpene derivative. The hydroxyl group in this compound is placed in an '' endo'' position. The exo diastereomer is called isoborneol. Being chiral, borneol exists as enantiomers, both of which are foun ...
chloride. In one of the proposed reaction mechanisms depicted in the paper, the positive charge of an intermediate cation was not assigned to a single atom but rather to the structure as a whole. This was later cited by opponents of the non-classical description as the first time that a non-classical ion was invoked. However, the term "non-classical ion" did not explicitly appear in the chemistry literature until over a decade later, when it was used to label delocalized bonding in a pyramidal, butyl cation.
The term ''synartetic ion'' was also invoked to describe delocalized bonding in stable carbocations before the term ''non-classical ion'' was in widespread use. The first users of this term commented on the striking similarity between bonding in these types of cations and bonding in borohydrides
Borohydride refers to the anion , which is also called tetrahydroborate or more commonly tetrahydrobiopterin, and its salts. Borohydride or hydroborate is also the term used for compounds containing , where ''n'' is an integer from 0 to 3, for ex ...
.
First non-classical proposals
In 1949,Saul Winstein
Saul Winstein (October 8, 1912 – November 23, 1969) was a Jewish Canadian chemist who discovered the ''Winstein reaction.'' He argued a non-classical cation was needed to explain the stability of the norbornyl cation. This fueled a debate ...
observed that 2-''exo''-norbornyl brosylate (''p''-bromobenzenesulfonate) and 2-''endo''-norbornyl tosylate
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl ...
(''p''-toluenesulfonate) gave a racemic mixture
In chemistry, a racemic mixture or racemate () is a mixture that has equal amounts (50:50) of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as r ...
of the same product, 2-''exo''-norbornyl acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
, upon acetolysis
In chemistry, solvolysis is a type of nucleophilic substitution (S1/S2) or elimination where the nucleophile is a solvent molecule. Characteristic of S1 reactions, solvolysis of a chiral reactant affords the racemate. Sometimes however, the ster ...
(''see Figure 6''). Since tosylates and brosylates work equally well as leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
s, he concluded that both the 2-''endo'' and 2-''exo'' substituted norbornane must be going through a common cationic intermediate with a dominant ''exo'' reactivity. He reported that this intermediate was most likely a symmetric, delocalized 2-norbornyl cation. It was later shown via vapor phase chromatography that the amount of the ''endo'' epimer
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization is t ...
of product produced was less than 0.02%, proving the high stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation ...
of the reaction.

 When a single
When a single enantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...
of 2-''exo''-norbornyl brosylate undergoes acetolysis, no optical activity is seen in the resulting 2-''exo''-norbornyl acetate (''see Figure 7''). Under the non-classical description of the 2-norbornyl cation, the plane of symmetry present (running through carbons 4, 5, and 6) allow equal access to both enantiomers of the product, resulting in the observed racemic mixture
In chemistry, a racemic mixture or racemate () is a mixture that has equal amounts (50:50) of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as r ...
.
It was also observed that the 2-''exo''-substituted norbornanes reacted 350 times faster than the corresponding ''endo'' isomers. Anchimeric assistance of the sigma bond between carbons 1 and 6 was rationalized as the explanation for this kinetic effect. Importantly, the invoked anchimeric assistance
In organic chemistry, neighbouring group participation (NGP, also known as anchimeric assistance) has been defined by the International Union of Pure and Applied Chemistry (IUPAC) as the interaction of a reaction centre with a lone pair of electro ...
led many chemists to postulate that the energetic stability of the 2-norbornyl cation was directly due to the symmetric, bridged structure invoked in the non-classical explanation. However, some other authors offered alternative explanations for the high stability without invoking a non-classical structure.
In 1951, it was first suggested that the 2-norbornyl cation could actually be better described when viewed as a nortricyclonium ion. It has been shown that the major product formed from an elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
of the 2-norbornyl cation is nortricyclene (not norbornene), but this has been claimed to support both non-classical ion postulates.
Herbert C. Brown: a dissenting view
Herbert C. Brown proposed that it was unnecessary to invoke a new type of bonding in stable intermediates to explain the reactivity of the 2-norbornyl cation. Criticizing many chemists for disregarding past explanations of reactivity, Brown argued that all of the aforementioned information about the 2-norbornyl cation could be explained using simplesteric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformational isomerism, co ...
present in the norbornyl system. Given that an alternative explanation using a rapidly equilibrating pair of ions for describing the 2-norbornyl cation was valid, he saw no need to invoke a stable, non-classical depiction of bonding. Invoking stable non-classical ions was becoming commonplace; Brown felt that this was not only unwarranted but also counterproductive for the field of chemistry as a whole. Indeed, many papers reporting stable non-classical ions were later retracted for being unrealistic or incorrect. After publishing this controversial view in 1962, Brown began a quest to find experimental evidence incompatible with the delocalized picture of bonding in the 2-norbornyl cation.
Brown also worked to prove the instability of a delocalized electronic structure for the 2-norbornyl cation. If the non-classical ion could be proven to be higher in energy than the corresponding classical ion pair, the non-classical ion would only be seen as a transition state between the two asymmetric cations. Though he did not rule out the possibility of a delocalized transition state Brown continued to reject the proposed reflectional symmetry of the 2-norbornyl cation, even late in his career.
Impact
The introduction of the three-centered two-electron delocalized bond invoked in the non-classical picture of the 2-norbornyl cation allowed chemists to explore a whole new realm of chemical bonds. Chemists were eager to apply the characteristics of hypovalent electronic states to new and old systems alike (though several got too carried away). One of the most fundamentally important concepts that emerged from the intense research focused around non-classical ions was the idea that electrons already involved in sigma bonds could be involved with reactivity. Though filled pi orbitals were known to beelectron donors
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up and down quarks.
El ...
, chemists had doubted that sigma orbitals could function in the same capacity. The non-classical description of the 2-norbornyl cation can be seen as the donation of an electron pair
In chemistry, an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the concepts of both the electron pair and the covalent bond in a landmark paper ...
from a carbon-carbon sigma bond into an empty p-orbital of carbon 2. Thus this carbocation showed that sigma-bond electron donation is as plausible as pi-bond electron donation.
The intense debate that followed Brown’s challenge to non-classical ion proponents also had a large impact on the field of chemistry. In order to prove or disprove the non-classical nature of the 2-norbornyl cation, chemists on both sides of the debate zealously sought out new techniques for chemical characterization and more innovative interpretations of existing data. One spectroscopic technique that was further developed to investigate the 2-norbornyl cation was nuclear magnetic resonance spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a Spectroscopy, spectroscopic technique based on re-orientation of Atomic nucleus, atomic nuclei with non-zero nuclear sp ...
of compounds in highly acidic media. Comparisons of the 2-norbornyl cation to unstable transition states with delocalized electronic states were often made when trying to elucidate whether the norbornyl system was stable or not. These efforts motivated closer investigations of transition states and vastly increased the scientific community’s understanding of their electronic structure. In short, vigorous competition between scientific groups led to an extensive research and a better understanding of the underlying chemical concepts.
Formation
The 2-norbornyl cation can be made by a multitude of synthetic routes. These routes can be grouped into three classes: ''σ Formation'', ''π Formation'', and ''Formation by Rearrangement''. Each of these is discussed separately below.
σ formation
The starting material for this route is a norbornane derivative with a good leaving group in position 2. If the leaving group is on the ''exo''- face, electron density from the ''σ bond'' between carbons 1 and 6 is donated into the ''σ*antibond
In theoretical chemistry, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more nodes ...
'' between carbon 2 and the leaving group (''see Figure 8b'').
If the leaving group is on the ''endo''- face, the leaving group first leaves on its own. Then electron density from the ''σ bond'' between carbons 1 and 6 is donated into the resulting empty atomic orbital on carbon 2. However, this formation route is much slower than that of the ''exo''- isomer because the σ bond cannot provide anchimeric assistance
In organic chemistry, neighbouring group participation (NGP, also known as anchimeric assistance) has been defined by the International Union of Pure and Applied Chemistry (IUPAC) as the interaction of a reaction centre with a lone pair of electro ...
for the first step, making the activation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
to the first transition state much higher. Additionally, if there is a high concentration of reactive electrophiles in the reaction mixture, formation of a newly substituted norbornane derivative may preclude non-classical ion formation.
An example of this formation route is the reaction that led Winstein and Trifan to propose the delocalized structure of the 2-norbornyl cation. 2-norbornyl tosylates and brosylates form the 2-norbornyl cation through this route as an intermediate towards solvolysis
In chemistry, solvolysis is a type of nucleophilic substitution (S1/S2) or elimination reaction, elimination where the nucleophile is a solvent molecule. Characteristic of S1 reactions, solvolysis of a chirality (chemistry), chiral reactant affor ...
.
π formation
The starting material for this route is a β-(Δ-cyclopentenyl)-ethane derivative with a good leaving group on the terminal carbon of the ethane group. Electron density from the ''π bond'' of the alkene moiety is donated into the σ* anti-bond between the terminal carbon and the leaving group (''see Figure 8c''). For example, the major product of the acetolysis of β-(Δ-cyclopentenyl)-ethyl nosylate (''p''-nitrobenzenesulfonate) is 2-''exo''-norbornyl acetate. The dearth of β-(Δ-cyclopentenyl)-ethyl acetate present after the reaction is explained by the greater stability of the norbornyl system over the decorated cyclopentenyl system. This route is only effective if the cyclopentenylolefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
is isolated from any larger π-bonded system. The reaction rate significantly decreases if the involved double bond forms a six-membered aromatic ring as it does in 2-indanylethyl nosylate. Alkyl substitutions on the olefins have been seen to increase the reaction rate by stabilizing the resulting carbocation.
Formation from rearrangement of 1-norbornyl and 7-norbornyl cations
The 2-norbornyl cation can also be formed via rearrangements of similar ions, such as the 1-norbornyl and 7-norbornyl cations, though these are generally not as well understood. Carbon-14 radioactive isotope labeling experiments have shown that complex scrambling in norbornyl cation systems allow C to be present at all seven positions of the norbornyl system. By cycling between low and high temperatures during the hydrolyses of 1- and 7-chloronorbornanes, a large amount of 2-norbornanol was observed in addition to the expected 1- and 7-norbornanols, respectively. Thus the 1- and 7-norbornyl cations have some mechanism by which they can rearrange to the more stable 2-norbornyl cation on the timescale of solvolysis reactions.Geometry
Spectroscopic evidence
One probe for testing whether or not the 2-norbornyl cation is non-classical is investigating the inherent symmetry of the cation. Many spectroscopic tools, such asnuclear magnetic resonance spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a Spectroscopy, spectroscopic technique based on re-orientation of Atomic nucleus, atomic nuclei with non-zero nuclear sp ...
(NMR spectroscopy) and Raman spectroscopy
Raman spectroscopy () (named after physicist C. V. Raman) is a Spectroscopy, spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Ra ...
, give hints about the reflectional and rotational
Rotation or rotational/rotary motion is the circular movement of an object around a central line, known as an ''axis of rotation''. A plane figure can rotate in either a clockwise or counterclockwise sense around a perpendicular axis intersec ...
symmetry present in a molecule or ion. Each of the three proposed structures of the 2-norbornyl cation illustrates a distinct molecular symmetry. The non-classical form contains a reflection plane through carbons 4, 5, 6, and the midpoint of carbons 1 and 2. The classical form contains neither reflectional nor rotational symmetry. The protonated nortricyclene structure contains a C-symmetric rotation axis through carbon 4.
Each peak in an NMR spectrum corresponds to a set of a particular element's atoms that are in similar chemical environments. The NMR spectrum of the antimony chloropentafluoride salt of the 2-norbornyl cation is not helpful at room temperature because hydride shifts occur faster than the timescale of an NMR experiment; most of the hydrogens are thus seen as equivalent and are accounted for in the same absorption peak. By lowering the temperature of the NMR experiment to −60 °C, hydride shifts are "frozen out" and more structural information can be gleaned from the spectrum. Researchers found that at these low temperatures, the H NMR spectrum matched what would be expected for the non-classical structure of the ion.
H and C NMR studies were able to confirm that any proposed Wagner-Meerwein rearrangements occurred faster than the timescale of the NMR experiment, even at low temperatures. For molecules in static equilibrium with respect to rearrangements, NMR reveals how many sets of symmetry-related nuclei are in the molecule and how many nuclei each of these sets accounts for via spectrum integration. For molecules in dynamic equilibrium such as the 2-norbornyl cation, nuclei within each set can also be transformed to one another through rearrangements with fast reaction rates. Since the proposed dynamic equilibrium of the classical ion proponents had very fast rates of rearrangement, the first NMR studies did not favor nor invalidate any of the three proposed structures. But by using solid-state NMR
Solid-state nuclear magnetic resonance (ssNMR) is a spectroscopy technique used to characterize atomic-level structure and dynamics in solid materials. ssNMR spectra are broader due to nuclear spin interactions which can be categorized as dipolar ...
analysis, one can lower the temperature of the NMR experiment to and thus significantly slow down any rearrangement phenomena. Solid-state C NMR spectra of the 2-norbornyl cation shows that carbons 1 and 2 are in identical chemical environments, which is consistent only with the non-classical picture of the 2-norbornyl cation.
Raman spectra of the 2-norbornyl cation show a more symmetric species than would be expected for a pair of rapidly equilibrating classical ions. Since the proposed reaction rates for the classical ion rearrangements are slower than the Raman timescale, one would expect the Raman spectra to indicate a less symmetric species if the classical picture were correct.
Some studies of the C NMR in particular favored interpretation via the protonated nortricyclene structure. In addition, Raman spectra of the 2-norbornyl cation in some acidic solvents show an absorption band at 3110 cm indicative of an electron-depleted cyclopropane ring. Since that absorption band would be expected in the C-symmetric protonated nortricyclene, some scientists claimed this as convincing evidence for this interpretation. Other chemists have postulated that the properties of the 2-norbornyl cation are very dependent on the solvent environment. Though the high acidity and low nucleophilicity of the solvents used in aforementioned experiments may cause the protonated nortricylconium geometry to be the most stable, this geometry need not be the most energetically favorable in other solvents.
Calculations and thermodynamics
Many calculations have been conducted on the classical vs nonclassical structures. Comparing the rearrangement between the 3-methyl-2-norbornyl cation and the 2-methyl-2-norbornyl cation to that between the tertiary and secondaryisopentane
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon (an alkane) with five carbon atoms, with formula or .
Isopentane is a volatile and flammable liquid. It is one of three structural isomers with t ...
carbocations, one finds that the change in enthalpy is about 6 kcal/mol less for the norbornyl system. Since the major difference between these two reversible rearrangements is the amount of delocalization possible in the electronic ground state, one can attribute the stabilization of the 3-methyl-2-norbornyl cation to its non-classical nature. However, some experimental studies failed to observe this stabilization in solvolysis reactions.
Other studies on the stability of the 2-norbornyl cation have shown that the alkyl substitutions at carbon 1 or 2 force the system to be decidedly classical. Tertiary carbocations are much more stable than their secondary counterparts and therefore do not need to adopt delocalized bonding in order to reach the lowest possible potential energy.
Kinetics
To support their suggestion of the non-classical nature of the 2-norbornyl cation, Winstein and Trifan first used kinetic evidence of the increased reaction rate for formation of the 2-''exo''-norbornyl cation over the 2-''endo''-norbornyl cation. Other researchers investigated the reaction rate of compounds that could feature anchimeric assistance but could not undergo rearrangements as the norbornyl system could show similar trends in rate enhancement. This has been claimed by some to be definitive evidence for the non-classical picture. But not all agree. Other researchers found that cyclopentane derivatives that were structurally similar to the norbornyl system still featured enhanced reaction rates, leading them to claim that the classical norbornyl cation describes the system much better.Isotope labeling experiments
 Radioactive isotope labeling experiments provide a powerful tool for determining the structure of organic molecules. By systematically decomposing the 2-norbornyl cation and analyzing the amount of radioactive isotope in each decomposition product, researchers were able to show further evidence for the non-classical picture of delocalized bonding (''see Figure 9''). Proponents of the nonclassical picture would expect 50% of the generated CO2 in the decomposition in Figure 9 to contain 14C, while proponents of the classical picture would expect more of the generated CO2 to be radioactive due to the short-lived nature of the cation. 40% of the carbon dioxide produced via decomposition has been observed to be radioactive, suggesting that the non-classical picture is more correct.
Further distinction between non-classical and classical structures of the 2-norbornyl cation is possible by combining NMR experiments with isotope-labeling experiments. Isotopic substitution of one of two deuterium atoms for a hydrogen atom causes the environment of nearby NMR-active atoms to change dramatically. Asymmetric deuterium isotope labeling (substitution) will cause a set of carbons that were all equivalent in the all-hydrogen species to be split into two or more sets of equivalent carbons in the deutero-labeled species; this will be manifested in the NMR spectrum as one peak in the all-hydrogen species' spectrum becoming at least two "split" peaks in the deutero-labeled species. If a system is undergoing a rapid equilibrium at a rate faster than the timescale of a C NMR experiment, the relevant peak will be split dramatically (on the order of 10-100 ppm). If the system is instead static, the peak will be split very little. The C NMR spectrum of the 2-norbornyl cation at -150 °C shows that the peaks corresponding to carbons 1 and 2 are split by less than 10 ppm (parts per million) when this experiment is carried out, indicating that the system is not undergoing a rapid equilibrium as in the classical picture.
Radioactive isotope labeling experiments provide a powerful tool for determining the structure of organic molecules. By systematically decomposing the 2-norbornyl cation and analyzing the amount of radioactive isotope in each decomposition product, researchers were able to show further evidence for the non-classical picture of delocalized bonding (''see Figure 9''). Proponents of the nonclassical picture would expect 50% of the generated CO2 in the decomposition in Figure 9 to contain 14C, while proponents of the classical picture would expect more of the generated CO2 to be radioactive due to the short-lived nature of the cation. 40% of the carbon dioxide produced via decomposition has been observed to be radioactive, suggesting that the non-classical picture is more correct.
Further distinction between non-classical and classical structures of the 2-norbornyl cation is possible by combining NMR experiments with isotope-labeling experiments. Isotopic substitution of one of two deuterium atoms for a hydrogen atom causes the environment of nearby NMR-active atoms to change dramatically. Asymmetric deuterium isotope labeling (substitution) will cause a set of carbons that were all equivalent in the all-hydrogen species to be split into two or more sets of equivalent carbons in the deutero-labeled species; this will be manifested in the NMR spectrum as one peak in the all-hydrogen species' spectrum becoming at least two "split" peaks in the deutero-labeled species. If a system is undergoing a rapid equilibrium at a rate faster than the timescale of a C NMR experiment, the relevant peak will be split dramatically (on the order of 10-100 ppm). If the system is instead static, the peak will be split very little. The C NMR spectrum of the 2-norbornyl cation at -150 °C shows that the peaks corresponding to carbons 1 and 2 are split by less than 10 ppm (parts per million) when this experiment is carried out, indicating that the system is not undergoing a rapid equilibrium as in the classical picture.
See also
*Carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
* Nonclassical Ions
References
External links
Nobel prize non-classical ion
{{DEFAULTSORT:Norbornyl cation, 2- Carbocations Physical organic chemistry Reactive intermediates