Weinreb Ketone Synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Weinreb ketone synthesis or Weinreb–Nahm ketone synthesis is a chemical reaction used in  The major advantage of this method over addition of organometallic reagents to more typical acyl compounds is that it avoids the common problem of over-addition. For these latter reactions, two equivalents of the incoming group add to form an
The major advantage of this method over addition of organometallic reagents to more typical acyl compounds is that it avoids the common problem of over-addition. For these latter reactions, two equivalents of the incoming group add to form an  The Weinreb–Nahm amide has since been adopted into regular use by organic chemists as a dependable method for the synthesis of ketones. These
The Weinreb–Nahm amide has since been adopted into regular use by organic chemists as a dependable method for the synthesis of ketones. These
 This chelation is in contrast to the mechanism for formation of the over-addition product wherein collapse of the tetrahedral intermediate allows a second addition. The mechanistic conjecture on the part of Weinreb was immediately accepted by the academic community, but it was not until 2006 that it was confirmed by spectroscopic and kinetic analyses.
This chelation is in contrast to the mechanism for formation of the over-addition product wherein collapse of the tetrahedral intermediate allows a second addition. The mechanistic conjecture on the part of Weinreb was immediately accepted by the academic community, but it was not until 2006 that it was confirmed by spectroscopic and kinetic analyses.
 A variety of
A variety of  Finally, an aminocarbonylation reaction reported by Stephen Buchwald allows conversion of
Finally, an aminocarbonylation reaction reported by Stephen Buchwald allows conversion of 
 Nonetheless, the Weinreb–Nahm amide figures prominently into many syntheses, serving as an important coupling partner for various fragments. Shown below are key steps involving Weinreb amides in the synthesis of several natural products, including members of the
Nonetheless, the Weinreb–Nahm amide figures prominently into many syntheses, serving as an important coupling partner for various fragments. Shown below are key steps involving Weinreb amides in the synthesis of several natural products, including members of the 
 Additionally, a one-pot magnesium–halogen exchange with subsequent arylation has been developed, showcasing the stability of the Weinreb–Nahm amide and providing an operationally simple method for the synthesis of aryl ketones.
Additionally, a one-pot magnesium–halogen exchange with subsequent arylation has been developed, showcasing the stability of the Weinreb–Nahm amide and providing an operationally simple method for the synthesis of aryl ketones.
 More unusual reagents with multiple Weinreb–Nahm amide functional groups have been synthesized, serving as CO2 and α-diketone
More unusual reagents with multiple Weinreb–Nahm amide functional groups have been synthesized, serving as CO2 and α-diketone 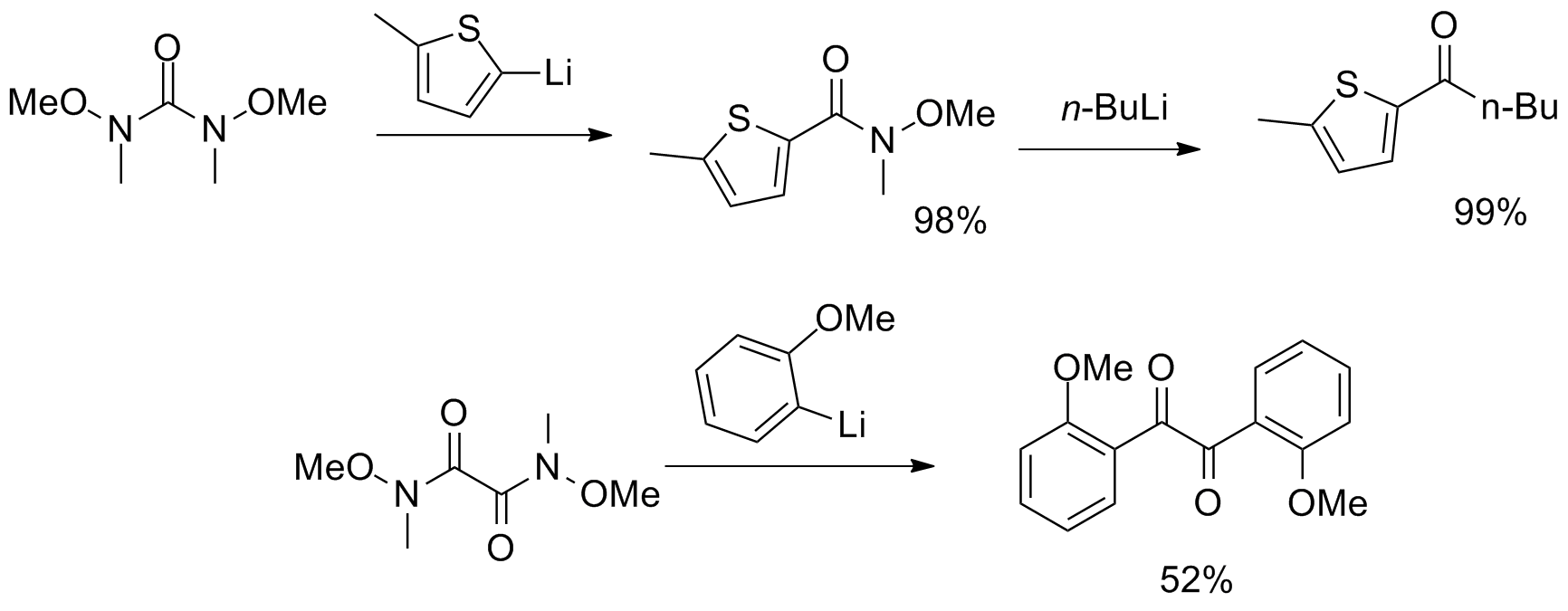 Finally,
Finally, 
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
to make carbon–carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed between on ...
s. It was discovered in 1981 by Steven M. Weinreb and Steven Nahm as a method to synthesize ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
. The original reaction involved two subsequent substitutions: the conversion of an acid chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
with ''N'',''O''-dimethylhydroxylamine, to form a Weinreb–Nahm amide, and subsequent treatment of this species with an organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
reagent such as a Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
or organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
. Nahm and Weinreb also reported the synthesis of aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s by reduction of the amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
with an excess of lithium aluminum hydride (see amide reduction
Amide reduction is a reaction in organic synthesis where an amide is reduced to either an amine or an aldehyde functional group.
Catalytic hydrogenation
Catalytic hydrogenation can be used to reduce amides to amines; however, the process often re ...
).
alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
rather than a ketone or aldehyde. This occurs even if the equivalents of nucleophile are closely controlled.
functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s are present in a large number of natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
s and can be reliably reacted to form new carbon–carbon bonds or converted into other functional groups. This method has been used in a number of syntheses, including macrosphelides A and B, amphidinolide J, and spirofungins A and B.
Mechanism
Weinreb and Nahm originally proposed the followingreaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
to explain the selectivity shown in reactions of the Weinreb–Nahm amide. Their suggestion was that the tetrahedral intermediate
A tetrahedral intermediate is a reaction intermediate in which the bond arrangement around an initially double-bonded carbon atom has been transformed from trigonal to tetrahedral. Tetrahedral intermediates result from nucleophilic addition to a c ...
(A below) formed as a result of nucleophilic addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic addit ...
by the organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
reagent is stabilized by chelation
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
from the methoxy
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula .
On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position a ...
group as shown. This intermediate is stable only at low temperatures, requiring a low-temperature quench
In materials science, quenching is the rapid cooling of a workpiece in water, gas, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such ...
.
Preparation
In addition to the original procedure shown above (which may have compatibility issues for sensitive substrates), Weinreb amides can be synthesized from a variety ofacyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group () or hydrogen in the case of formyl grou ...
compounds. The vast majority of these procedures utilize the commercially available salt N,O-dimethylhydroxylamine hydrochloride eO(Me)NH•HCl
Eo or EO may refer to:
Businesses and organizations
* Education Otherwise, a home education organization
* Elevorganisasjonen, a Norwegian student organization
* Entrepreneurs' Organization, a nonprofit network
* Evangelische Omroep, a public ...
which is typically easier to handle than the free amine.
Treatment of an ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
or lactone
Lactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated.
Lactones are formed by lactonization, the intramolecular esterification of the corresp ...
with AlMe3 or AlMe2Cl affords the corresponding Weinreb amide in good yields. Alternatively, non-nucleophilic Grignard reagents such as isopropyl magnesium chloride can be used to activate the amine before addition of the ester.
 A variety of
A variety of peptide coupling
In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl ...
reagents can also be used to prepare Weinreb–Nahm amides from carboxylic acids. Various carbodiimide
In organic chemistry, a carbodiimide (systematic IUPAC name: methanediimine) is a functional group with the formula RN=C=NR. On Earth they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its ...
-, hydroxybenzotriazole
Hydroxybenzotriazole (abbreviated HOBt) is an organic compound with the formula . It is a derivative of benzotriazole. It is a white crystalline powder, which as a commercial product contains some water (~11.7% wt as the HOBt monohydrate crystal ...
-, and triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a l ...
-based couplings have been reported specifically for this purpose.
 Finally, an aminocarbonylation reaction reported by Stephen Buchwald allows conversion of
Finally, an aminocarbonylation reaction reported by Stephen Buchwald allows conversion of aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
halides directly into aryl Weinreb–Nahm amides.

Scope
The standard conditions for the Weinreb–Nahm ketone synthesis are known to tolerate a wide variety of functional groups elsewhere in the molecule, including alpha-halogen substitution, N-protectedamino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s, α-β unsaturation, silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protectin ...
s, various lactams
A lactam is a cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words '' lactone'' + ''amide''.
Nomenclature
Greek prefixes in alphabetical order indicate ring size.
...
and lactones, sulfonates
In organosulfur chemistry, a sulfonate is a salt, anion or ester of a sulfonic acid. Its formula is , containing the functional group , where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of ...
, sulfinates, and phosphonate esters. A wide variety of nucleophiles can be used in conjunction with the amide. Lithiates and Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
s are most commonly employed; examples involving aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all ...
, vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
, aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
, and alkynyl
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
carbon nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
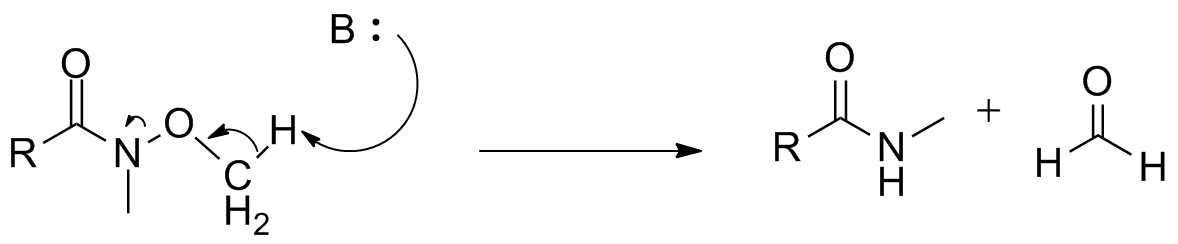
s have been reported. However, with highly basic or sterically hindered nucleophiles, elimination of the methoxide moiety to release formaldehyde can occur as a significant side reaction.
 Nonetheless, the Weinreb–Nahm amide figures prominently into many syntheses, serving as an important coupling partner for various fragments. Shown below are key steps involving Weinreb amides in the synthesis of several natural products, including members of the
Nonetheless, the Weinreb–Nahm amide figures prominently into many syntheses, serving as an important coupling partner for various fragments. Shown below are key steps involving Weinreb amides in the synthesis of several natural products, including members of the immunosuppressant
Immunosuppressive drugs, also known as immunosuppressive agents, immunosuppressants and antirejection medications, are drugs that inhibit or prevent the activity of the immune system.
Classification
Immunosuppressive drugs can be classified ...
family of macrosphelides, and the antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...
family of spirofungins.

Variations
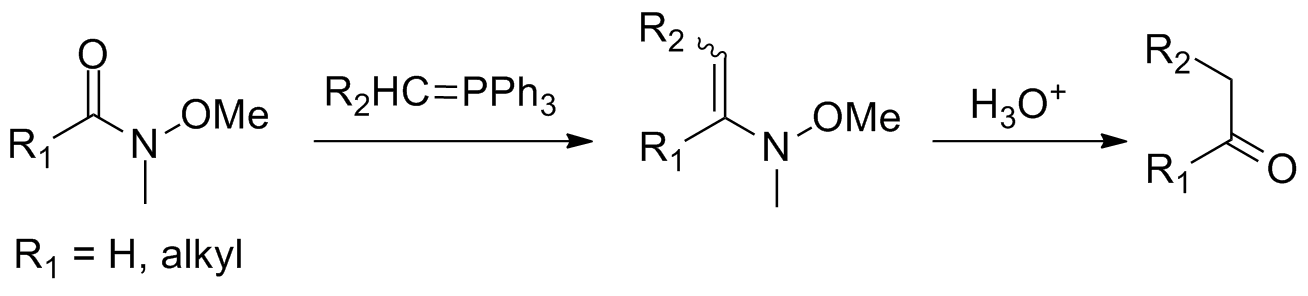
Reaction of Weinreb–Nahm amides withWittig reagents In organic chemistry, Wittig reagents are organophosphorus compounds of the formula R3P=CHR', where R is usually phenyl. They are used to convert ketones and aldehydes to alkenes:
:
Preparation
Because they typically hydrolyze and oxidize readily, ...
has been performed to avoid the sometimes harsh conditions required for addition of hydride reagents or organometallic compounds. This yields an N-methyl-N-methoxy-enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and the r ...
that converts to the corresponding ketone or aldehyde upon hydrolytic workup.
 Additionally, a one-pot magnesium–halogen exchange with subsequent arylation has been developed, showcasing the stability of the Weinreb–Nahm amide and providing an operationally simple method for the synthesis of aryl ketones.
Additionally, a one-pot magnesium–halogen exchange with subsequent arylation has been developed, showcasing the stability of the Weinreb–Nahm amide and providing an operationally simple method for the synthesis of aryl ketones.
 More unusual reagents with multiple Weinreb–Nahm amide functional groups have been synthesized, serving as CO2 and α-diketone
More unusual reagents with multiple Weinreb–Nahm amide functional groups have been synthesized, serving as CO2 and α-diketone synthon
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1988 ...
s.
 Finally,
Finally, Stephen G. Davies
Stephen Graham Davies (born 24 February 1950) is a British chemist and was, until his retirement, the Waynflete Professor of Chemistry at the University of Oxford.
Education
Davies obtained his Bachelor of Arts degree in 1973 from New Coll ...
of Oxford
Oxford () is a City status in the United Kingdom, cathedral city and non-metropolitan district in Oxfordshire, England, of which it is the county town.
The city is home to the University of Oxford, the List of oldest universities in continuou ...
has designed a chiral auxiliary
In stereochemistry, a chiral auxiliary is a Stereogenic center, stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxil ...
that combines the functionality of the Weinreb amide with that of the Myers' pseudoephedrine
Pseudoephedrine, sold under the brand name Sudafed among others, is a sympathomimetic medication which is used as a decongestant to treat nasal congestion. It has also been used off-label for certain other indications, like treatment of lo ...
auxiliary, allowing diastereoselective enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the Organic synthesis, synthesis of organic compounds.
Bonding and structure
Enolate ...
alkylation followed by facile cleavage to the corresponding enantioenriched aldehyde or ketone.

See also
*N,O-Dimethylhydroxylamine
''N'',''O''-Dimethylhydroxylamine is a methylated hydroxylamine used to form so called 'Weinreb amides' for use in the Weinreb ketone synthesis. It is commercially available as its hydrochloride salt.
Synthesis
It may be prepared by reacting e ...
* Ketone#Synthesis
References
{{DEFAULTSORT:Weinreb Ketone Synthesis Carbon-carbon bond forming reactions Substitution reactions Name reactions