Trisilaallene on:
[Wikipedia]
[Google]
[Amazon]
 Trisilaallene is a subclass of
Trisilaallene is a subclass of
 Sekiguchi ''et al.'' synthesized a
Sekiguchi ''et al.'' synthesized a 
 In contrast to its linear carbon analog, trisilaallene is characterized by bent geometry. For example, Kira's trisilaallene had a Si=Si=Si
In contrast to its linear carbon analog, trisilaallene is characterized by bent geometry. For example, Kira's trisilaallene had a Si=Si=Si
 DFT calculation at B3LYP/6-31+G(d, p) level suggests that the frontier molecular orbitals of the tetraalkyl-substituted trisilaallene are markedly different from those of allene. The calculation was performed on a model trisilaallene compound with methyl substituents and the experimentally observed bond angle from Kira's compound (136.5º). According to the calculation, the alkyl-substituted trisilaallene has nondegenerate
DFT calculation at B3LYP/6-31+G(d, p) level suggests that the frontier molecular orbitals of the tetraalkyl-substituted trisilaallene are markedly different from those of allene. The calculation was performed on a model trisilaallene compound with methyl substituents and the experimentally observed bond angle from Kira's compound (136.5º). According to the calculation, the alkyl-substituted trisilaallene has nondegenerate  The electronic structure of trisilaallene is highly affected by its substituents. On the contrary to the alkyl-substituted trisilaallene, according to DFT calculation at B3LYP/6-31G(d) level, ''t''Bu2MeSi-substituted trisilaallene has almost degenerate π (Si=Si) and π* (Si=Si) orbitals, localized between the central silicon atom and only one of the two terminal silicon atoms. The two π-bonding orbitals and the two π*-antibonding orbitals are perpendicular to each other. These features are analogous to all-carbon allenes, which is justified by the close-to-linear geometry of the silyl-substituted trisilaallene.
The electronic structure of trisilaallene is highly affected by its substituents. On the contrary to the alkyl-substituted trisilaallene, according to DFT calculation at B3LYP/6-31G(d) level, ''t''Bu2MeSi-substituted trisilaallene has almost degenerate π (Si=Si) and π* (Si=Si) orbitals, localized between the central silicon atom and only one of the two terminal silicon atoms. The two π-bonding orbitals and the two π*-antibonding orbitals are perpendicular to each other. These features are analogous to all-carbon allenes, which is justified by the close-to-linear geometry of the silyl-substituted trisilaallene.
 Trisilaallene readily reacts with alcohol to generate dialkoxytrisilane. The regioselectivity of the alcohol addition reaction depends on the type of substituents. In 2007, Kira ''et al.'' reported that the alkyl-substituted trisilaallene gives rise to 1,3-dialkoxytrisilane in the presence of excess ROH (R = H, Me, Et). Larger alcohols such as isopropanol and ''tert''-butanol did not react due to the steric congestion arising from the bulky substituents of trisilaallene. In contrast, two methoxy groups were added to the central silicon atom of the silyl-substituted trisilaallene from the reaction with methanol.
Trisilaallene readily reacts with alcohol to generate dialkoxytrisilane. The regioselectivity of the alcohol addition reaction depends on the type of substituents. In 2007, Kira ''et al.'' reported that the alkyl-substituted trisilaallene gives rise to 1,3-dialkoxytrisilane in the presence of excess ROH (R = H, Me, Et). Larger alcohols such as isopropanol and ''tert''-butanol did not react due to the steric congestion arising from the bulky substituents of trisilaallene. In contrast, two methoxy groups were added to the central silicon atom of the silyl-substituted trisilaallene from the reaction with methanol.
 The different regioselectivity between the alkyl-substituted trisilaallene and the silyl-substituted trisilaallene is explained by charge distribution within Si=Si=Si unit. Calculation at the B3LYP/6-31+G(d,p) level for the methyl-substituted trisilaallene predicted >Siδ+=Siδ-=Siδ+< charge distribution with -0.408 on the central atom and +1.016 / +1.026 on the terminal atoms. Therefore, the nucleophilic addition of alcohol occurs at the terminal atoms. This charge distribution is in accordance with the frontier molecular orbitals of the alkyl-substituted trisilaallene. HOMO-1 and HOMO have the largest orbital coefficient on the central atom, while LUMO and LUMO+1 have larger coefficients on the terminal two. On the other hand, the silyl-substituted trisilaallene is expected to have greater negative charges on the terminal atoms (-0.36 / -0.37) than on the central atom (-0.08), calculated at B3LYP/6-31G(d) level.
The different regioselectivity between the alkyl-substituted trisilaallene and the silyl-substituted trisilaallene is explained by charge distribution within Si=Si=Si unit. Calculation at the B3LYP/6-31+G(d,p) level for the methyl-substituted trisilaallene predicted >Siδ+=Siδ-=Siδ+< charge distribution with -0.408 on the central atom and +1.016 / +1.026 on the terminal atoms. Therefore, the nucleophilic addition of alcohol occurs at the terminal atoms. This charge distribution is in accordance with the frontier molecular orbitals of the alkyl-substituted trisilaallene. HOMO-1 and HOMO have the largest orbital coefficient on the central atom, while LUMO and LUMO+1 have larger coefficients on the terminal two. On the other hand, the silyl-substituted trisilaallene is expected to have greater negative charges on the terminal atoms (-0.36 / -0.37) than on the central atom (-0.08), calculated at B3LYP/6-31G(d) level.
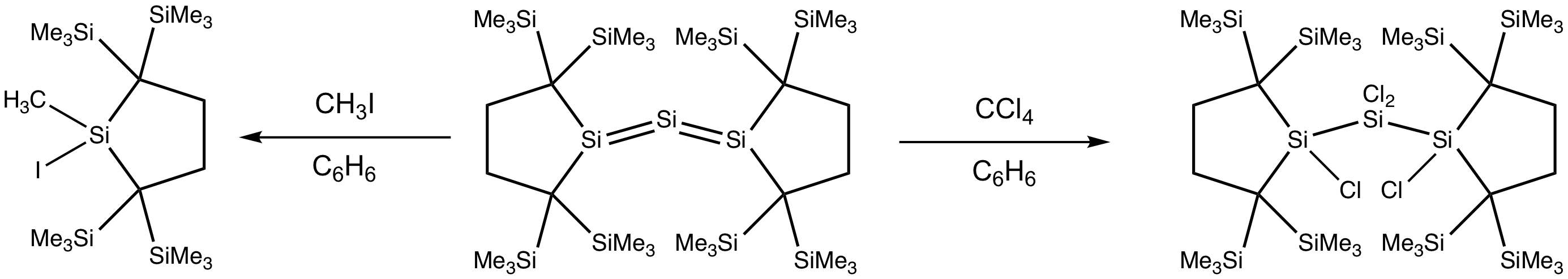 Reactions of trisilaallene with haloalkanes generate halogenated silane products. Treating trisilaallene with methyl iodide breaks the Si=Si double bonds, yielding two molecules of iodo(methyl)silane. Meanwhile, the reaction of trisilaallene with tetrachloromethane gives rise to the complete chlorination of the two Si=Si bonds without bond breaking, yielding tetrachlorotrisilane.
:
Reactions of trisilaallene with haloalkanes generate halogenated silane products. Treating trisilaallene with methyl iodide breaks the Si=Si double bonds, yielding two molecules of iodo(methyl)silane. Meanwhile, the reaction of trisilaallene with tetrachloromethane gives rise to the complete chlorination of the two Si=Si bonds without bond breaking, yielding tetrachlorotrisilane.
: Trisilaallene also reacts with acetone to form a strained bicyclic adduct, whose structure was confirmed by X-ray crystallography. This reaction is supposed to be initiated by an ene reaction of one of the Si=Si double bond with acetone, followed by + 2cycloaddition of the other Si=Si bond and a C=C bond to give the product.
:
Trisilaallene also reacts with acetone to form a strained bicyclic adduct, whose structure was confirmed by X-ray crystallography. This reaction is supposed to be initiated by an ene reaction of one of the Si=Si double bond with acetone, followed by + 2cycloaddition of the other Si=Si bond and a C=C bond to give the product.
: The silyl-substituted trisilaallene goes through thermal rearrangement when heated to 120 °C in benzene. The resultant isomer, , was calculated to be thermodynamically more stable than the parent trisilaallene compound by 10.5 kcal/mol.
The silyl-substituted trisilaallene goes through thermal rearrangement when heated to 120 °C in benzene. The resultant isomer, , was calculated to be thermodynamically more stable than the parent trisilaallene compound by 10.5 kcal/mol.
 Trisilaallene is a subclass of
Trisilaallene is a subclass of silenes
In inorganic chemistry, silenes, or disilalkenes,Philip P. Power "pi-Bonding and the Lone Pair Effect in Multiple Bonds between Heavier Main Group Elements" Chemical Reviews, 1999, 99, 3462. are silicon compounds that contain double bonds, w ...
derivatives where a central silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
atom forms double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
s with each of two terminal silicon atoms, with the generic formula R2Si=Si=SiR2. Trisilaallene is a silicon-based analog of an allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms (, where R is hydrogen, H or some organyl group). Allenes are classified as diene#Classes, cumulated dienes ...
, but their chemical properties are markedly different.
Synthesis
The first isolable trisilaallene compound was reported by Kira ''et al.'' in 2003, synthesized by reductive dehalogenation of tetrachlorosilane usingpotassium graphite
In the area of solid state chemistry, graphite intercalation compounds are a family of materials prepared from graphite. In particular, the sheets of carbon that comprise graphite can be pried apart by the insertion ( intercalation) of ions. Th ...
. This tetraalkyl-substituted trisilallene showed thermal stability up to its melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
around 200 °C, but decomposed in contact with air. Its remarkable stability is attributable to bulky substituents providing kinetic protection at the terminal silicon atoms. The 29Si-NMR shift
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic fi ...
s of the central silicon and terminal silicon atoms were observed at 157.0 ppm and 196.9 ppm respectively. Two Si=Si bond lengths were determined to be 2.177 Å and 2.188 Å by X-ray crystallography, which are within the typical range of Si=Si double bonds.
: Sekiguchi ''et al.'' synthesized a
Sekiguchi ''et al.'' synthesized a silyl
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. Silylations are core methods for production of organosilicon chemistry. Silanization, while similar to silylation, usually refers to attachmen ...
-substituted trisilaallene from a reaction between a N-heterocyclic carbene (NHC) adduct of SiCl2 and 1,1-dilithiosilane (''t''-Bu2MeSi)2SiLi2. Although crystallographic analysis of the product was not successful, the formation of trisilaallene was confirmed by 1H-, 13C-, and 29Si-NMR spectroscopy, high-resolution mass spectrometry (HRMS), and reactivity study. The low electronegativity of silyl substituents compared to alkyl substituents resulted in a more upfield 29Si-NMR shift for the terminal silicon atoms (44.6 ppm) and a downfield shift for the central atom (418.5 ppm).
:
Structure and bonding
Geometry
 In contrast to its linear carbon analog, trisilaallene is characterized by bent geometry. For example, Kira's trisilaallene had a Si=Si=Si
In contrast to its linear carbon analog, trisilaallene is characterized by bent geometry. For example, Kira's trisilaallene had a Si=Si=Si bond angle
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that deter ...
of 136.5º. While the bulky and electropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
silyl ( ''t''Bu2MeSi-) substituents widened the bond angle to 164.3º (calculated), no linear trisilaallene has been reported yet. The two planes that each terminal silicon atom and attached substituents lie on tend to be perpendicular to each other, which is analogous with allene. The central silicon atom shows fluxional
In chemistry and molecular physics, fluxional (or non-rigid) molecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in som ...
behavior in that its relative position varies with respect to the substituents planes, and the distribution of resultant isomers depends on the temperature.
The bent structure of trisilaallene is explained by the second-order Jahn-Teller distortion. Unlike the 2s and 2p orbital
Orbital may refer to:
Sciences Chemistry and physics
* Atomic orbital
* Molecular orbital
* Hybrid orbital Astronomy and space flight
* Orbit
** Earth orbit
Medicine and physiology
* Orbit (anatomy), also known as the ''orbital bone''
* Orbitof ...
s of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, where the orbital radii of maximum electron density are similar, the 3s orbital of silicon is significantly smaller than 3p orbitals (rnp– rns= -0.2 pm for n = 2 and > 20 pm for n > 2). Therefore, the σ-overlap between the 3s orbital of the central silicon atom and a set of 3pz orbitals of the terminal atoms (when z-axis is the molecular axis) is poor compared to in allene, resulting in a relatively low-lying σ*-orbital. The energy gap between σ*-orbital and π-orbitals originated from 3px and 3py orbitals is small enough to induce considerable mixing between the σ*-orbital and one of the π-orbitals with appropriate symmetry. This orbital mixing removes degeneracy between the two π-orbitals, accompanied by geometric distortion.
The ab initio
( ) is a Latin term meaning "from the beginning" and is derived from the Latin ("from") + , ablative singular of ("beginning").
Etymology
, from Latin, literally "from the beginning", from ablative case of "entrance", "beginning", related t ...
and density functional theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
(DFT) calculations predict that Si3R4 molecules with smaller substituents (R = H or Me) adopt zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively and negatively charged functional groups.
:
(1,2- dipolar compounds, such as ylides, are sometimes excluded from ...
ic structures with C2v or Cs symmetry and with drastically smaller bond angles (~70º for R = H, ~90º for R = Me). However, the steric congestion required for isolation prevent these highly bent structures.
Frontier molecular orbitals
 DFT calculation at B3LYP/6-31+G(d, p) level suggests that the frontier molecular orbitals of the tetraalkyl-substituted trisilaallene are markedly different from those of allene. The calculation was performed on a model trisilaallene compound with methyl substituents and the experimentally observed bond angle from Kira's compound (136.5º). According to the calculation, the alkyl-substituted trisilaallene has nondegenerate
DFT calculation at B3LYP/6-31+G(d, p) level suggests that the frontier molecular orbitals of the tetraalkyl-substituted trisilaallene are markedly different from those of allene. The calculation was performed on a model trisilaallene compound with methyl substituents and the experimentally observed bond angle from Kira's compound (136.5º). According to the calculation, the alkyl-substituted trisilaallene has nondegenerate HOMO
''Homo'' () is a genus of great ape (family Hominidae) that emerged from the genus ''Australopithecus'' and encompasses only a single extant species, ''Homo sapiens'' (modern humans), along with a number of extinct species (collectively called ...
-1 and HOMO based on π-interaction and also nondegenerate LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
and LUMO+1 based on π*-interaction, as a direct result of Jahn-Teller distortion. These orbitals correspond to in-plane and out-of-plane twisted overlaps of p-orbitals, which are delocalized over the Si=Si=Si unit. These frontier orbitals make a striking contrast to those of all-carbon allenes, whose frontier orbitals consist of degenerate pairs of π-bonding orbitals (HOMO) and π*-antibonding orbitals (LUMO) localized between two carbons. The electronic structure of trisilaallene is highly affected by its substituents. On the contrary to the alkyl-substituted trisilaallene, according to DFT calculation at B3LYP/6-31G(d) level, ''t''Bu2MeSi-substituted trisilaallene has almost degenerate π (Si=Si) and π* (Si=Si) orbitals, localized between the central silicon atom and only one of the two terminal silicon atoms. The two π-bonding orbitals and the two π*-antibonding orbitals are perpendicular to each other. These features are analogous to all-carbon allenes, which is justified by the close-to-linear geometry of the silyl-substituted trisilaallene.
The electronic structure of trisilaallene is highly affected by its substituents. On the contrary to the alkyl-substituted trisilaallene, according to DFT calculation at B3LYP/6-31G(d) level, ''t''Bu2MeSi-substituted trisilaallene has almost degenerate π (Si=Si) and π* (Si=Si) orbitals, localized between the central silicon atom and only one of the two terminal silicon atoms. The two π-bonding orbitals and the two π*-antibonding orbitals are perpendicular to each other. These features are analogous to all-carbon allenes, which is justified by the close-to-linear geometry of the silyl-substituted trisilaallene.
Reactivity
Alcohol addition
: Trisilaallene readily reacts with alcohol to generate dialkoxytrisilane. The regioselectivity of the alcohol addition reaction depends on the type of substituents. In 2007, Kira ''et al.'' reported that the alkyl-substituted trisilaallene gives rise to 1,3-dialkoxytrisilane in the presence of excess ROH (R = H, Me, Et). Larger alcohols such as isopropanol and ''tert''-butanol did not react due to the steric congestion arising from the bulky substituents of trisilaallene. In contrast, two methoxy groups were added to the central silicon atom of the silyl-substituted trisilaallene from the reaction with methanol.
Trisilaallene readily reacts with alcohol to generate dialkoxytrisilane. The regioselectivity of the alcohol addition reaction depends on the type of substituents. In 2007, Kira ''et al.'' reported that the alkyl-substituted trisilaallene gives rise to 1,3-dialkoxytrisilane in the presence of excess ROH (R = H, Me, Et). Larger alcohols such as isopropanol and ''tert''-butanol did not react due to the steric congestion arising from the bulky substituents of trisilaallene. In contrast, two methoxy groups were added to the central silicon atom of the silyl-substituted trisilaallene from the reaction with methanol.
 The different regioselectivity between the alkyl-substituted trisilaallene and the silyl-substituted trisilaallene is explained by charge distribution within Si=Si=Si unit. Calculation at the B3LYP/6-31+G(d,p) level for the methyl-substituted trisilaallene predicted >Siδ+=Siδ-=Siδ+< charge distribution with -0.408 on the central atom and +1.016 / +1.026 on the terminal atoms. Therefore, the nucleophilic addition of alcohol occurs at the terminal atoms. This charge distribution is in accordance with the frontier molecular orbitals of the alkyl-substituted trisilaallene. HOMO-1 and HOMO have the largest orbital coefficient on the central atom, while LUMO and LUMO+1 have larger coefficients on the terminal two. On the other hand, the silyl-substituted trisilaallene is expected to have greater negative charges on the terminal atoms (-0.36 / -0.37) than on the central atom (-0.08), calculated at B3LYP/6-31G(d) level.
The different regioselectivity between the alkyl-substituted trisilaallene and the silyl-substituted trisilaallene is explained by charge distribution within Si=Si=Si unit. Calculation at the B3LYP/6-31+G(d,p) level for the methyl-substituted trisilaallene predicted >Siδ+=Siδ-=Siδ+< charge distribution with -0.408 on the central atom and +1.016 / +1.026 on the terminal atoms. Therefore, the nucleophilic addition of alcohol occurs at the terminal atoms. This charge distribution is in accordance with the frontier molecular orbitals of the alkyl-substituted trisilaallene. HOMO-1 and HOMO have the largest orbital coefficient on the central atom, while LUMO and LUMO+1 have larger coefficients on the terminal two. On the other hand, the silyl-substituted trisilaallene is expected to have greater negative charges on the terminal atoms (-0.36 / -0.37) than on the central atom (-0.08), calculated at B3LYP/6-31G(d) level.
Miscellaneous
: Reactions of trisilaallene with haloalkanes generate halogenated silane products. Treating trisilaallene with methyl iodide breaks the Si=Si double bonds, yielding two molecules of iodo(methyl)silane. Meanwhile, the reaction of trisilaallene with tetrachloromethane gives rise to the complete chlorination of the two Si=Si bonds without bond breaking, yielding tetrachlorotrisilane.
:
Reactions of trisilaallene with haloalkanes generate halogenated silane products. Treating trisilaallene with methyl iodide breaks the Si=Si double bonds, yielding two molecules of iodo(methyl)silane. Meanwhile, the reaction of trisilaallene with tetrachloromethane gives rise to the complete chlorination of the two Si=Si bonds without bond breaking, yielding tetrachlorotrisilane.
: Trisilaallene also reacts with acetone to form a strained bicyclic adduct, whose structure was confirmed by X-ray crystallography. This reaction is supposed to be initiated by an ene reaction of one of the Si=Si double bond with acetone, followed by + 2cycloaddition of the other Si=Si bond and a C=C bond to give the product.
:
Trisilaallene also reacts with acetone to form a strained bicyclic adduct, whose structure was confirmed by X-ray crystallography. This reaction is supposed to be initiated by an ene reaction of one of the Si=Si double bond with acetone, followed by + 2cycloaddition of the other Si=Si bond and a C=C bond to give the product.
: The silyl-substituted trisilaallene goes through thermal rearrangement when heated to 120 °C in benzene. The resultant isomer, , was calculated to be thermodynamically more stable than the parent trisilaallene compound by 10.5 kcal/mol.
The silyl-substituted trisilaallene goes through thermal rearrangement when heated to 120 °C in benzene. The resultant isomer, , was calculated to be thermodynamically more stable than the parent trisilaallene compound by 10.5 kcal/mol.
See also
*Allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms (, where R is hydrogen, H or some organyl group). Allenes are classified as diene#Classes, cumulated dienes ...
*Silenes
In inorganic chemistry, silenes, or disilalkenes,Philip P. Power "pi-Bonding and the Lone Pair Effect in Multiple Bonds between Heavier Main Group Elements" Chemical Reviews, 1999, 99, 3462. are silicon compounds that contain double bonds, w ...
*Organosilicon
Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, f ...
References
{{Reflist Organosilicon compounds