Trimethylenemethane Cycloaddition on:
[Wikipedia]
[Google]
[Amazon]
Trimethylenemethane cycloaddition is the formal (3+2)
 The rapid closure of TMMs to methylidenecyclopropanes is a general problem that affects the rate and yield of (3+2) cycloaddition reactions involving this class of
The rapid closure of TMMs to methylidenecyclopropanes is a general problem that affects the rate and yield of (3+2) cycloaddition reactions involving this class of 
 When stabilizing groups are present, MCPs may open to the corresponding zwitterionic TMMs. Acetal 1 has been used in this context, and provides
When stabilizing groups are present, MCPs may open to the corresponding zwitterionic TMMs. Acetal 1 has been used in this context, and provides  MCPs lacking stabilizing groups may generate TMM synthons in the presence of
MCPs lacking stabilizing groups may generate TMM synthons in the presence of 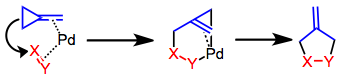 Silylated allylic acetates,
Silylated allylic acetates, 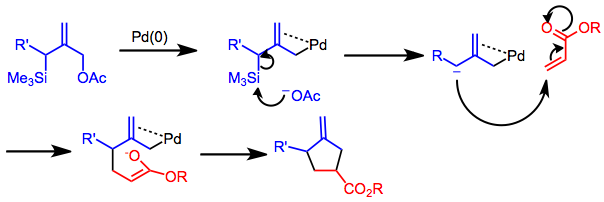
 In reactions of silyl-substituted allylic acetates, chiral
In reactions of silyl-substituted allylic acetates, chiral 
 Usually, unless a cyclic pi system is involved TMM cycloadditions exhibit 2π periselectivity and do not react with larger pi systems. Polar MCPs, for example, react only with the 2,3 double bond of polyunsaturated
Usually, unless a cyclic pi system is involved TMM cycloadditions exhibit 2π periselectivity and do not react with larger pi systems. Polar MCPs, for example, react only with the 2,3 double bond of polyunsaturated  Transition-metal catalyzed reactions have the potential to quickly generate an interesting functionality.
Transition-metal catalyzed reactions have the potential to quickly generate an interesting functionality.  Silylated allylic acetates may be employed for intra- or intermolecular applications.
Silylated allylic acetates may be employed for intra- or intermolecular applications.  Polarized trimethylenemethanes generated from polar MCPs are also useful substrates for (3+2) reactions with polar double bonds as the 2π component.
Polarized trimethylenemethanes generated from polar MCPs are also useful substrates for (3+2) reactions with polar double bonds as the 2π component. 


annulation
In organic chemistry, annulation (; occasionally annelation) is a chemical reaction in which a new ring is constructed on a molecule.
:
Examples are the Robinson annulation, Danheiser annulation and certain cycloadditions. Annular molecules a ...
of trimethylenemethane
Trimethylenemethane (often abbreviated TMM) is a chemical compound with molecular formula, formula . It is a Charge (chemistry), neutral free molecule with two unsatisfied valence bonds, and is therefore a highly reactive free radical. Formally, ...
(TMM) derivatives to two-atom pi system
In mathematics, a -system (or pi-system) on a set \Omega is a collection P of certain subsets of \Omega, such that
* P is non-empty.
* If A, B \in P then A \cap B \in P.
That is, P is a non-empty family of subsets of \Omega that is closed und ...
s. Although TMM itself is too reactive and unstable to be stored, reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
s which can generate TMM or TMM synthon
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1988 ...
s ''in situ'' can be used to effect cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
reactions with appropriate electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. Electron acceptors are oxidizing agents.
The electron accepting power of an electron acceptor is measured by its redox potential.
In the ...
s. Generally, electron-deficient pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
s undergo cyclization
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where ...
with TMMs more easily than electron-rich pi bonds.
Introduction
Trimethylenemethane is a neutral, four-carbon molecule composed of four pi bonds; thus, it must be expressed either as anon-Kekulé molecule
A non-Kekulé molecule is a conjugated system, conjugated hydrocarbon that cannot be assigned a classical Kekulé structure.
Since non-Kekulé molecules have two or more formal charges or
radical (chemistry), radical centers, their Spin (physics ...
or a zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively and negatively charged functional groups.
:
(1,2- dipolar compounds, such as ylides, are sometimes excluded from ...
. The orbital energy levels of TMM reveal that it possesses singlet and triplet state
In quantum mechanics, a triplet state, or spin triplet, is the quantum state of an object such as an electron, atom, or molecule, having a quantum spin ''S'' = 1. It has three allowed values of the spin's projection along a given axis ''m''S = � ...
s; generally, these states exhibit different reactivity and selectivity
Selectivity may refer to:
Psychology and behaviour
* Choice, making a selection among options
* Discrimination, the ability to recognize differences
* Socioemotional selectivity theory, in social psychology
Engineering
* Selectivity (radio), a ...
profiles. A singlet (3+2) cycloaddition, when it is concerted, is believed to proceed under frontier orbital
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
control. When electron-rich TMMs are involved, the ''A'' orbital serves as the HOMO (leading to fused products if the TMM is cyclic). When electron-poor (or unsubstituted) TMMs are involved, the ''S'' orbital serves as the HOMO (leading to bridged
Bridging may refer to:
Construction
* Building of bridges across a gap
* Cross bracing used between joists to stabilize them
Electronics and computing
* In electronics, using a low source impedance to drive a large load impedance for maximum vol ...
products if the TMM is cyclic). Cycloadditions involving the triplet state are stepwise, and usually result in configurational scrambling in the two-atom component.
 The rapid closure of TMMs to methylidenecyclopropanes is a general problem that affects the rate and yield of (3+2) cycloaddition reactions involving this class of
The rapid closure of TMMs to methylidenecyclopropanes is a general problem that affects the rate and yield of (3+2) cycloaddition reactions involving this class of reaction intermediate
In chemistry, a reaction intermediate, or intermediate, is a molecular entity arising within the sequence of a stepwise chemical reaction. It is formed as the reaction product of an elementary step, from the reactants and/or preceding interme ...
s. The problem is generally less severe for five-membered, cyclic TMMs due to ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles ar ...
in the corresponding MCPs. When ring closure
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where ...
and TMM dimerization
In chemistry, dimerization is the process of joining two identical or similar molecular entities by bonds. The resulting bonds can be either strong or weak. Many symmetrical chemical species are described as dimers, even when the monomer is u ...
can be controlled, (3+2) cycloaddition affords isomeric mixtures of methylenecyclopentanes. Three classes of compounds have been used to generate synthetically useful TMM intermediates: diazene
Diimide, also called diazene or diimine, is a compound having the formula HN=NH. It exists as two geometric isomers, ''E'' (''trans'') and ''Z'' (''cis''). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an ...
s, silyl-substituted allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
ic acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
s and methylenecyclopropenes. Transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
can be used with the latter two classes, although polar MCPs may open under light or heat (see below).

Mechanism and stereochemistry
Prevailing mechanisms
Diazenes may extrude nitrogen to provide discrete TMM intermediates. Generally, bridged diazenes are used to avoid competitive closure to MCPs and dimerization reactions. In combination with an alkenic acceptor, cyclization to either fused or bridged products takes place. Fused products are generally favored, unless the diazene precursor is substituted with electron-donating groups at the methylene carbon atom. The configuration of the alkene is maintained as long as the reaction is proceeding through a singlet TMM. When stabilizing groups are present, MCPs may open to the corresponding zwitterionic TMMs. Acetal 1 has been used in this context, and provides
When stabilizing groups are present, MCPs may open to the corresponding zwitterionic TMMs. Acetal 1 has been used in this context, and provides cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic compound, alicyclic hydrocarbon with chemical formula C5H10, C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and ...
s with the acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...
functionality ''exo'' to the newly formed ring with high selectivity. This reaction is also stereospecific
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one (or a subset) of the stereoisomers."Overlap C ...
with respect to alkene geometry, and exhibits high selectivity for ''endo'' products in most cases.
 MCPs lacking stabilizing groups may generate TMM synthons in the presence of
MCPs lacking stabilizing groups may generate TMM synthons in the presence of palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
(0) or nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
(0) catalysts. Formal insertion of the catalyst into either of the two chemically distinct cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
bonds (called "distal" and "proximal" to reflect their distance from the double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
) has the potential to generate isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
ic products. Generally, palladium catalysts cause formal distal bond cleavage. This process is believed to occur through direct attack of the distal bond on the coordinated alkene. The reaction is stepwise and lacks stereospecificity under both palladium and nickel catalysis.
 Silylated allylic acetates,
Silylated allylic acetates, carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
s and other substituted allyl compounds may form TMM synthons under palladium catalysis. The reaction is highly regioselective
In organic chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a str ...
, providing only the substitution pattern shown below regardless of the position of the R' group on the starting allylic acetate. However, cyclization takes place in a stepwise fashion and does not exhibit stereospecificity. Rapid racemization
In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. This creates a 1:1 molar ratio of enantiomers and is referred to as a racemic mixture (i.e. cont ...
of chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
pi-allyl palladium complexes occurs, and only moderate diastereoselectivity
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have d ...
is observed in reactions of chiral allylic acetates. Chiral non-racemic alkenes, however, may exhibit moderate to high diastereoselectivity.

Stereoselective variants
Chiral auxiliaries
In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the st ...
on the alkene partner have been used for stereoselective transformations. In the reaction of camphorsultam-derived unsaturated amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s, lower temperatures were needed to achieve high selectivities.
 In reactions of silyl-substituted allylic acetates, chiral
In reactions of silyl-substituted allylic acetates, chiral sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s can be used to enforce high diastereofacial selectivity.

Scope and limitations
The primary limitations of TMM cycloadditions employing diazenes are competitive MCP and dimer formation. To circumvent these problems, either very high concentrations of alkene must be used or the cycloaddition must be intramolecular. Stereoselectivity and site selectivity may also be higher in intramolecular variants of cycloadditions starting from diazenes. Usually, unless a cyclic pi system is involved TMM cycloadditions exhibit 2π periselectivity and do not react with larger pi systems. Polar MCPs, for example, react only with the 2,3 double bond of polyunsaturated
Usually, unless a cyclic pi system is involved TMM cycloadditions exhibit 2π periselectivity and do not react with larger pi systems. Polar MCPs, for example, react only with the 2,3 double bond of polyunsaturated ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s.
 Transition-metal catalyzed reactions have the potential to quickly generate an interesting functionality.
Transition-metal catalyzed reactions have the potential to quickly generate an interesting functionality. Propellane
In organic chemistry, propellane is any member of a class of polycyclic compound, polycyclic hydrocarbons, whose carbon skeleton consists of three rings of carbon atoms sharing a common carbon–carbon bond, carbon–carbon covalent bond. The co ...
s have been generated from intramolecular cyclization under palladium catalysis.
 Silylated allylic acetates may be employed for intra- or intermolecular applications.
Silylated allylic acetates may be employed for intra- or intermolecular applications. Carbonyl compounds
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes ...
may be used as the 2π component under the appropriate conditions. For instance, in the presence of an indium
Indium is a chemical element; it has Symbol (chemistry), symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are la ...
co-catalyst, the reactive 2π component of the cycloaddition below switches from the C-C to the C-O double bond.
 Polarized trimethylenemethanes generated from polar MCPs are also useful substrates for (3+2) reactions with polar double bonds as the 2π component.
Polarized trimethylenemethanes generated from polar MCPs are also useful substrates for (3+2) reactions with polar double bonds as the 2π component. Orthoester
In organic chemistry, an ortho ester is a functional group containing three alkoxy groups attached to one carbon atom, i.e. with the general formula . Orthoesters may be considered as products of exhaustive alkylation of unstable orthocarboxylic ...
products are generally favored over ketene acetals.

Synthetic applications
The high stereospecificity and stereoselectivity inherent in many TMM cycloaddition reactions is a significant advantage; for instance, the ''trans'' ring junction in TMM cycloaddition adduct 2 was carried through in a synthesis of (+)-brefeldin A
Brefeldin A is a lactone antiviral produced by the fungus '' Penicillium brefeldianum''. Brefeldin A inhibits protein transport from the endoplasmic reticulum to the golgi complex indirectly by preventing association of COP-I coat to the Golgi ...
.

Comparison with other methods
Although 1,3-dipolar cycloaddition is a useful method for the generation of five-memberedheterocyclic compounds
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, proper ...
, few methods exist to synthesize five-membered carbocyclic rings in a single step via annulation. Most of these, like TMM cycloaddition, rely on the generation of a suitable three-atom component for combination with a stable two-atom partner such as an alkene or alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
. When heated, cyclopropene acetals rearrange to vinylcarbenes, which can serve as the three-atom component in cycloadditions with highly electron-deficient alkenes. Zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
homoenolates can also serve as indirect three-atom components, and undergo cyclization to cyclopentenones in the presence of an unsaturated ester. Tandem intermolecular-intramolecular cyclization of homopropargylic radicals leads to methylenecyclopropane
Methylenecyclopropane is an organic compound with the formula . It is a hydrocarbon which, as the name suggests, is derived from the addition of a Methylene group, methylene () substituent to a cyclopropane ring. It is a colourless, easily conden ...
s.Curran, D. P.; Chen, M.-H. ''J. Am. Chem. Soc.'' 1987, ''109'', 6558.

Experimental conditions and procedure
Typical conditions
The optimal conditions for TMM cycloadditions depend on both the TMM source and two-atom component. However, a few general principles for each of the TMM sources have emerged. Reactions of diazenes should employ degassed solvents to avoid radical reactions with oxygen.Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
(THF) at reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to Chemical ...
is the most commonly employed solvent system, but photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons wi ...
conditions at low temperature may also be used.
Reactions employing polar MCPs are usually carried out in a polar solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
to facilitate formation of the TMM intermediate. Although rigorous exclusion of air and water is not required, it is generally preferred.
For transition-metal catalyzed MCP reactions, the choice of catalyst and ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
system is key. Generally, phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
or phosphite
The general structure of a phosphite ester showing the lone pairs on the P
In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of ...
ligands are required in conjunction with a palladium(0) or nickel(0) source; the most common are Pd2(dba)3 and Ni(cod)2. Tri(isopropyl)phosphine is the most common ligand used with palladium, and triarylphosphites are usually added in nickel-catalyzed reactions.
For transition-metal catalyzed reactions of silylated allylic acetates, the most commonly used catalyst system is palladium(II) acetate and tri(isopropyl)phosphite. Reactions are usually carried out in THF at temperatures ranging from 60 to 110 °C. The choice of solvent or leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
may affect the course of the reaction.
References
{{reflist, 30em Cycloadditions