Transition-metal Dichalcogenide on:
[Wikipedia]
[Google]
[Amazon]
: 220px, Cadmium sulfide, a prototypical metal chalcogenide, is used as a yellow pigment.
A chalcogenide is a chemical compound consisting of at least one
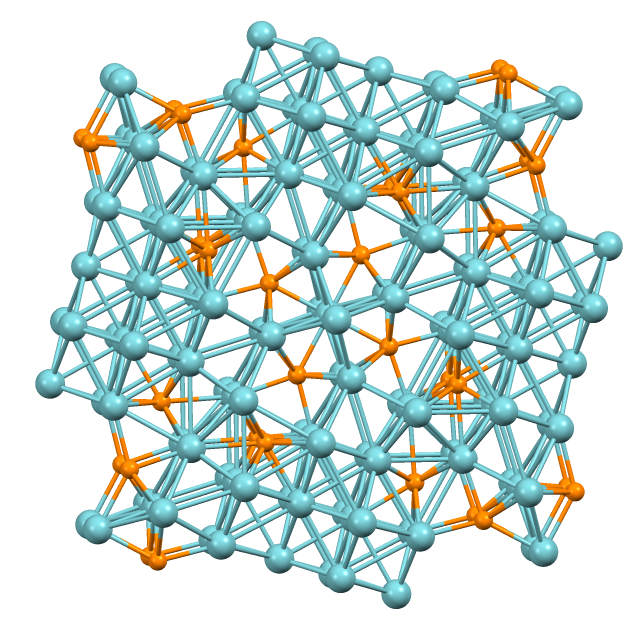 In most of their chalcogenides, transition metals adopt oxidation states of II or greater. Nonetheless, several examples exist where the metallic atoms far outnumber the chalcogens. Such compounds typically have extensive metal-metal bonding.
In most of their chalcogenides, transition metals adopt oxidation states of II or greater. Nonetheless, several examples exist where the metallic atoms far outnumber the chalcogens. Such compounds typically have extensive metal-metal bonding.
Advanced Chalcogenide Technologies and Applications Lab
''ACTAlab'' Jun 14, 2016
Phase change memory-based 'moneta' system points to the future of computer storage
''ScienceBlog'' Jun 03, 2011 *
Big Blue boffins hatch dirt-cheap solar cells
The Register, 12 February 2010 {{Authority control
chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
and at least one more electropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
element. Although all group 16 element
The chalcogens (ore forming) ( ) are the chemical elements in group (periodic table), group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellur ...
s of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s, selenide
A selenide is a chemical compound containing a selenium with oxidation number of −2. Similar to sulfide, selenides occur both as inorganic compounds and as organic derivatives, which are called organoselenium compound.
Inorganic selenides
Th ...
s, tellurides, and polonide
A polonide is a chemical compound of the radioactive element polonium with any element less electronegative than polonium. Polonides are usually prepared by a direct reaction between the elements at temperatures of around 300–400 °C... They a ...
s, rather than oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
s. Many metal ores exist as chalcogenides. Photoconductive
Photoconductivity is an optical and electrical phenomenon in which a material becomes more electrically conductive due to the absorption of electromagnetic radiation such as visible light, ultraviolet light, infrared light, or gamma radiation.
W ...
chalcogenide glass
Chalcogenide glass (pronounced hard ''ch'' as in ''chemistry'') is a glass containing one or more heavy chalcogens (sulfur, selenium or tellurium; polonium is also a heavy chalcogen but too radioactive to use). Chalcogenide materials behave rather ...
es are used in xerography
Xerography is a dry photocopying technique. Originally called electrophotography, it was renamed xerography—from the Greek roots , meaning "dry" and , meaning "writing"—to emphasize that unlike reproduction techniques then in use such as c ...
. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.
Alkali metal and alkaline earth chalcogenides
Alkali metal and alkaline earth monochalcogenides are salt-like, being colourless and often water-soluble. The sulfides tend to undergo hydrolysis to form derivatives containingbisulfide
Bisulfide (or bisulphide in British English) is an inorganic anion with the chemical formula HS− (also written as SH−). It contributes no color to bisulfide salts, and its salts may have a distinctive putrid smell. It is a strong base. Bisul ...
(SH−) anions. The alkali metal chalcogenides often crystallize with the antifluorite structure
The fluorite structure refers to a common motif for compounds with the formula MX2. The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic (FCC) structure. Many compounds, notabl ...
and the alkaline earth salts in the sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
motif.
:
Transition metal chalcogenides
Transition metal chalcogenides occur with many stoichiometries and many structures.Vaughan, D. J.; Craig, J. R. "Mineral Chemistry of Metal Sulfides" Cambridge University Press, Cambridge: 1978. . Most common and most important technologically, however, are the chalcogenides of simple stoichiometries, such as 1:1 and 1:2. Extreme cases include metal-rich phases (e.g. Ta2S), which exhibit extensive metal-metal bonding, and chalcogenide-rich materials such as Re2S7, which features extensive chalcogen-chalcogen bonding. For the purpose of classifying these materials, the chalcogenide is often viewed as a dianion, i.e., S2−, Se2−, Te2−, and Po2−. In fact, transition metal chalcogenides are highlycovalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
, not ionic, as indicated by their semiconducting properties.
Metal-rich chalcogenides
 In most of their chalcogenides, transition metals adopt oxidation states of II or greater. Nonetheless, several examples exist where the metallic atoms far outnumber the chalcogens. Such compounds typically have extensive metal-metal bonding.
In most of their chalcogenides, transition metals adopt oxidation states of II or greater. Nonetheless, several examples exist where the metallic atoms far outnumber the chalcogens. Such compounds typically have extensive metal-metal bonding.
Monochalcogenides
Metal monochalcogenides have the formula ME, where M = a transition metal and E = S, Se, Te. They typically crystallize in one of two motifs, named after the corresponding forms ofzinc sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various i ...
. In the zinc blende
Sphalerite is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-Valley type, and volcanogenic mas ...
structure, the sulfide atoms pack in a cubic symmetry and the Zn2+ ions occupy half of the tetrahedral holes. The result is a diamondoid
In chemistry, diamondoids are generalizations of the carbon cage molecule known as adamantane (C10H16), the smallest unit cage structure of the diamond crystal lattice. Diamondoids also known as nanodiamonds or condensed adamantanes may include one ...
framework. The main alternative structure for the monochalcogenides is the wurtzite
Wurtzite is a zinc and iron sulfide mineral with the chemical formula , a less frequently encountered Polymorphism (materials science), structural polymorph form of sphalerite. The iron content is variable up to eight percent.Palache, Charles, H ...
structure wherein the atom connectivities are similar (tetrahedral), but the crystal symmetry is hexagonal. A third motif for metal monochalcogenide is the nickel arsenide
Nickel arsenide is a compound of nickel and arsenic and component of the ore nickeline. It is highly toxic and a known carcinogen in humans. Uncontrolled decomposition of nickel arsenide can give rise to further toxic nickel compounds.
Toxicit ...
lattice, where the metal and chalcogenide each have octahedral and trigonal prismatic coordination, respectively. This motif is commonly subject to nonstoichiometry
Non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); most often, in su ...
.
Important monochalcogenides include some pigment
A pigment is a powder used to add or alter color or change visual appearance. Pigments are completely or nearly solubility, insoluble and reactivity (chemistry), chemically unreactive in water or another medium; in contrast, dyes are colored sub ...
s, notably cadmium sulfide
Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow salt.Egon Wiberg, Arnold Frederick Holleman (2001''Inorganic Chemistry'' Elsevier It occurs in nature with two different crystal structures as the rare min ...
. Many minerals and ores are monosulfides.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. .
Dichalcogenides
Metal dichalcogenides have the formula ME2, where M = a transition metal and E = S, Se, Te.Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. . The most important members are the sulfides. They are always dark diamagnetic solids, insoluble in all solvents, and exhibitsemiconducting
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping levels ...
properties. Some are superconductors
Superconductivity is a set of physical properties observed in superconductors: materials where electrical resistance vanishes and magnetic fields are expelled from the material. Unlike an ordinary metallic conductor, whose resistance decreases ...
.
In terms of their electronic structures, these compounds are usually viewed as derivatives of M4+, where M4+ = Ti4+ (d0 configuration), V4+ (d1 configuration), Mo4+ (d2 configuration). Titanium disulfide
Titanium disulfide is an inorganic compound with the formula Ti S2. A golden yellow solid with high electrical conductivity, it belongs to a group of compounds called transition metal di chalcogenides, which consist of the stoichiometry M E2. ...
was investigated in prototype cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
s for secondary batteries, exploiting its ability to reversibly undergo intercalation by lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
. Molybdenum disulfide is the subject of thousands of articles and the main ore of molybdenum, termed molybdenite
Molybdenite is a mineral of molybdenum disulfide, Mo S2. Similar in appearance and feel to graphite, molybdenite has a lubricating effect that is a consequence of its layered structure. The atomic structure consists of a sheet of molybdenum at ...
. It is used as a solid lubricant and catalyst for hydrodesulfurization
Hydrodesulfurization (HDS), also called hydrotreatment or hydrotreating, is a catalytic chemical process widely used to desulfurization, remove sulfur (S) from natural gas and from oil refinery, refined petroleum products, such as gasoline, g ...
. The corresponding diselenides and even ditellurides are known, e.g., TiSe2, MoSe2, and WSe2.
Transition metals
Transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
dichalcogenides typically adopt either cadmium diiodide or molybdenum disulfide
Molybdenum disulfide (or moly) is an inorganic chemistry, inorganic compound composed of molybdenum and sulfur. Its chemical formula is .
The compound is classified as a transition metal dichalcogenide. It is a silvery black solid that occurs as ...
structures. In the CdI2 motif, the metals exhibit octahedral structures. In the MoS2 motif, which is not observed for dihalides, the metals exhibit trigonal prismatic structures. The strong bonding between the metal and chalcogenide ligands, contrasts with the weak chalcogenide—chalcogenide bonding between the layers. Owing to these contrasting bond strengths, these materials engage in intercalation by alkali metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
. The intercalation process is accompanied by charge transfer, reducing the M(IV) centers to M(III). The attraction between electrons and holes in 2D tungsten diselenide is 100s of times stronger than in a typical 3D semiconductor.
Pyrite and related disulfides
In contrast to classical metal dichalcogenides,iron pyrite
The mineral pyrite ( ), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue ...
, a common mineral, is usually described as consisting of Fe2+ and the persulfido anion S22−. The sulfur atoms within the persulfido dianion are bound together via a short S-S bond. "Late" transition metal disulfides (Mn, Fe, Co, Ni) almost always adopt the pyrite or the related marcasite
The mineral marcasite, sometimes called "white iron pyrite", is iron sulfide (FeS2) with orthorhombic crystal structure. It is physically and crystallographically distinct from pyrite, which is iron sulfide with cubic crystal structure. Both ...
motif, in contrast to early metals (V, Ti, Mo, W) which adopt 4+ oxidation state with two chalcogenide dianions.
Tri- and tetrachalcogenides
Several metals, mainly for the early metals (Ti, V, Cr, Mn groups) also form trichalcogenides. These materials are usually described as M4+(E22−)(E2−) (where E = S, Se, Te). A well known example isniobium triselenide
Niobium triselenide is an inorganic compound belonging to the class of transition metal trichalcogenides. It has the formula NbSe3. It was the first reported example of one-dimensional compound to exhibit the phenomenon of sliding charge density ...
. Amorphous MoS3 is produced by treatment of tetrathiomolybdate with acid:
:MoS42− + 2 H+ → MoS3 + H2S
The mineral patrónite
Patrónite is the vanadium sulfide mineral with chemical formula, formula Vanadium, Vsulfur, S4. The material is usually described as V4+(S22−)2. Structurally, it is a "linear-chain" compound with alternating bonding and nonbonding contacts b ...
, which has the formula VS4, is an example of a metal tetrachalcogenide. Crystallographic analysis shows that the material can be considered a bis(persulfide), i.e. V4+,(S22−)2.
Main group chalcogenides
: Chalcogen derivatives are known for all of themain group element
In chemistry and atomic physics, the main group is the group (periodic table), group of chemical element, elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon ...
s except the noble gases. Usually, their stoichiometries follow the classical valence trends, e.g. SiS2, B2S3, Sb2S3. Many exceptions exist however, e.g. P4S3 and S4N4. The structures of many main group materials are dictated by directional covalent bonding, rather than by close packing.
The chalcogen is assigned positive oxidation states for the halides, nitrides, and oxides.
See also
* Carbon dichalcogenide *Chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
*Chalcogenide glass
Chalcogenide glass (pronounced hard ''ch'' as in ''chemistry'') is a glass containing one or more heavy chalcogens (sulfur, selenium or tellurium; polonium is also a heavy chalcogen but too radioactive to use). Chalcogenide materials behave rather ...
*Hydrogen chalcogenide Hydrogen chalcogenides (also chalcogen hydrides or hydrogen chalcides) are binary compounds of hydrogen with chalcogen atoms (elements of group 16: oxygen, sulfur, selenium, tellurium, polonium, and livermorium). Water, the first chemical compound i ...
*Negative resistance
In electronics, negative resistance (NR) is a property of some electrical circuits and devices in which an increase in voltage across the device's terminals results in a decrease in electric current through it.
This is in contrast to an ordina ...
*Phase-change memory
Phase-change memory (also known as PCM, PCME, PRAM, PCRAM, OUM (ovonic unified memory) and C-RAM or CRAM (chalcogenide RAM)) is a type of non-volatile random-access memory. PRAMs exploit the unique behaviour of chalcogenide glass. In PCM, heat pr ...
References
External links
Advanced Chalcogenide Technologies and Applications Lab
''ACTAlab'' Jun 14, 2016
Phase change memory-based 'moneta' system points to the future of computer storage
''ScienceBlog'' Jun 03, 2011 *
Big Blue boffins hatch dirt-cheap solar cells
The Register, 12 February 2010 {{Authority control