thiazoline on:
[Wikipedia]
[Google]
[Amazon]
Thiazolines (or dihydrothiazoles) are a group of
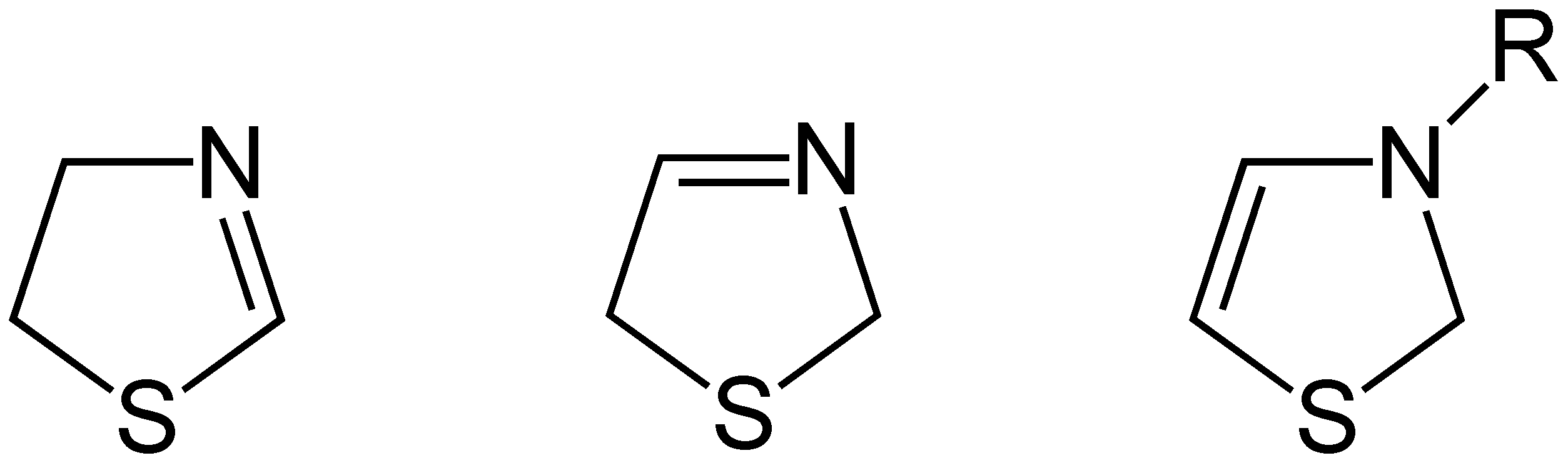 Three
Three
isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
...
ic 5-membered heterocyclic compounds containing both sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
and nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seve ...
in the ring. Although unsubstituted thiazolines are rarely encountered themselves, their derivatives
The derivative of a function is the rate of change of the function's output relative to its input value.
Derivative may also refer to:
In mathematics and economics
*Brzozowski derivative in the theory of formal languages
*Formal derivative, an ...
are more common and some are bioactive. For example, in a common post-translational modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribos ...
, cysteine residues are converted into thiazolines.
The name thiazoline originates from the Hantzsch–Widman nomenclature.
Isomers
 Three
Three structural isomers
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term met ...
of thiazoline exist depending on the position of the double bond. These forms do not readily interconvert and hence are not tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hyd ...
s. Of these 2-thiazoline is the most common.
A fourth structure exists in which the N and S atoms are adjacent; this known as isothiazoline.
Synthesis
Thiazolines were first prepared by dialkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effectin ...
of thioamide
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure R–CS–NR′R″, where R, R′, and R″ are organic groups. They are analogous to amides but they exhibit greater multiple bond ch ...
s by Richard Willstatter in 1909. 2-Thiazolines are commonly prepared from 2-aminoethanethiols (e.g. cysteamine). They may also be synthesized via the Asinger reaction.
Applications
Many molecules contain thiazoline rings, one example being Firefly luciferin, the light-emitting molecule in fireflies. The amino acidcysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, s ...
is produced industrially from substituted thiazole. 2-Aminothiazoline-4-carboxylic acid
2-Aminothiazoline-4-carboxylic acid (ACTA) is the organosulfur compound and a heterocycle with the formula HO2CCHCH2SCNH2N. This derivative of thiazoline is an intermediate in the industrial synthesis of L-cysteine, an amino acid. ACTA exists i ...
is an intermediate in the industrial synthesis of L-cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, s ...
.{{Ullmann, author=Karlheinz Drauz, Ian Grayson, Axel Kleemann, Hans-Peter Krimmer, Wolfgang Leuchtenberger, Christoph Weckbecker, year=2006, doi=10.1002/14356007.a02_057.pub2
See also
*Thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular fo ...
- an analogue with 2 double bonds
* Thiazolidine - an analogue with no double bonds
* Oxazoline - an analogue with O in place of S
References