|
Oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond. Oxazoline itself has no applications however oxazolines have been widely investigated for potential applications. These applications include use as ligands in asymmetric catalysis, as protecting groups for carboxylic acids and increasingly as monomers for the production of polymers. Isomers Synthesis The synthesis of 2-oxazoline rings is well established and in general proceeds via the cyclisation of a 2-amino alcohol (typically obtained by the reduction of an amino acid) with a suitable functional group. The overall mechanism is usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoxazoline
Isoxazolines are a class of five-membered heterocyclic chemical compounds, containing one atom each of oxygen and nitrogen which are located adjacent to one another. The ring was named in-line with the Hantzsch–Widman nomenclature. They are structural isomers of the more common oxazolines and exist in three different isomers depending on the location of the double bond. The relatively weak N-O bond makes isoxazolines prone to ring-opening and rearrangement reactions. Compounds containing an isoxazoline ring, sometimes referred to isoxazolyls, have a variety of uses with many being biologically active. A number of naturally occurring isoxazolines with possible anti-cancer activity are produced by marine sponges. Perhaps the most commonly encountered products containing isoxazolines are some veterinary medicines used to prevent flea infestations in dogs e.g. Fluralaner, Afoxolaner and Sarolaner. Synthesis 2-Isoxazolines are generally produced by the 1,3-dipolar cycloaddition o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Alcohol
In organic chemistry, alkanolamines are organic compounds that contain both hydroxyl () and amino (, , and ) functional groups on an alkane backbone. The term alkanolamine is a broad class term that is sometimes used as a subclassification. Methanolamine.svg, methanolamine, an intermediate in the reaction of ammonia with formaldehyde Ethanolamine.png, Ethanolamine 2-amino-2-methyl-1-propanol.svg, 2-amino-2-methyl-1-propanol is a precursor to oxazolines valinol.svg, valinol is derived from the amino acid valine Sphingosine structure.svg, Sphingosine is a component of some cell membrane. 1-Aminoalcohols 1-Aminoalcohols are better known as hemiaminals. Methanolamine is the simplest member. 2-Aminoalcohols Key members: ethanolamine, dimethylethanolamine, ''N''-methylethanolamine, Aminomethyl propanol Two popular drugs, often called alkanolamine beta blockers, are members of this structural class: propranolol, pindolol. Isoetarine is yet another medicinally useful ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminomethyl Propanol
Aminomethyl propanol is an organic compound with the formula H2NC(CH3)2CH2OH. It is colorless liquid that is classified as an alkanolamine. It is a useful buffer and a precursor to numerous other organic compounds. Synthesis Aminomethyl propanol can be produced by the hydrogenation of 2-aminoisobutyric acid or its esters. Properties Aminomethyl propanol is soluble in water and about the same density as water. Uses Aminomethyl propanol is used for the preparation of buffer solutions. It is a component of the drugs ambuphylline and pamabrom. It is also used in cosmetics. It is a precursor to oxazolines via its reaction with acyl chlorides. Via sulfation of the alcohol, the compound is also a precursor to 2,2-dimethylaziridine. It is used in the synthesis of Fepradinol & G-130. It is also used for Isobucaine, and Radafaxine Radafaxine (developmental code name GW-353,162), also known as (2''S'',3''S'')-hydroxybupropion or (''S'',''S'')-hydroxybupropion, is a norepinephrin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazolidine
An oxazolidine is a five-membered ring compound consisting of three carbon atoms, a nitrogen atom and an oxygen atom. The O atom and NH group are the 1 and 3 positions, respectively. In oxazolidine derivatives, there is always a carbon atom between the O and N atoms (or it would be an ''isoxazolidine''). All of the carbon atoms in oxazolidines are reduced (compare to oxazole and oxazoline). Some of their derivatives, the oxazolidinediones, are used as anticonvulsants. Oxazolidines were first synthesized over 100 years ago. Monooxazolidines Oxazolidines that are the precursor to bisoxazolidines are in effect mono-oxazolidines. They are also used as moisture scavengers in polyurethane and other systems. Dioxooxazolidines Oxazolidines where the carbon centers at the 1 and 3 positions are carbonyls are called dioxooxazolidines. Some of these are commercial fungicides including chlozolinate, vinclozolin, and famoxadone. Bisoxazolidines Bisoxazolidines are chemical compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymmetric Catalysis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereomeric) products in unequal amounts." Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer. Enantiomers are stereoisomers that have opposite configurations at every chiral center. Diastereomers are stereoisomers that differ at one or more chiral centers. Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity. Overview Many of the building blocks of biological systems such as sugars and amino acids are produced exclusively as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Appel Reaction
The Appel reaction is an organic reaction that converts an alcohol into an alkyl chloride using triphenylphosphine and carbon tetrachloride. The use of carbon tetrabromide or bromine as a halide source will yield alkyl bromides, whereas using carbon tetraiodide, methyl iodide or iodine gives alkyl iodides. The reaction is credited to and named after Rolf Appel, it had however been described earlier. The use of this reaction is becoming less common, due to carbon tetrachloride being restricted under the Montreal protocol. Drawbacks to the reaction are the use of toxic halogenating agents and the coproduction of organophosphorus product that must be separated from the organic product. The phosphorus reagent can be used in catalytic quantities. The corresponding alkyl bromide can also be synthesised by addition of lithium bromide as a source of bromide ions. A greener, more sustainable catalytic Appel reaction, free from chlorinated solvents, has also been reported. Mechanism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thionyl Chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons. Thionyl chloride is sometimes confused with sulfuryl chloride, , but the properties of these compounds differ significantly. Sulfuryl chloride is a source of chlorine whereas thionyl chloride is a source of chloride ions. Production The major industrial synthesis involves the reaction of sulfur trioxide and sulfur dichloride: This synthesis can be adapted to the laboratory by heating oleum to slowly distill the sulfur trioxide into a cooled flask of sulfur dichloride. :SO3 + SCl2 -> SOCl2 + SO2 Other methods i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much lar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions. Structure For ketimines and aldimines, respectively, the five core atoms (C2C=NX and C(H)C=NX, X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29-1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C-N distances in amines and nitriles are 1.47 and 1.16 Å, respectively. Rotation about the C=N bond is slow. Using NMR spectroscopy, both E- and Z-isomers of aldimines have been detected. Owing to steric effects, the E isomer is favored. Nomenclature and classification The term "imine" was coin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalyl Chloride
Oxalyl chloride is an organic chemical compound with the formula (COCl)2. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis. Preparation Oxalyl chloride was first prepared in 1892 by the French chemist Adrien Fauconnier, who reacted diethyl oxalate with phosphorus pentachloride. It can also be prepared by treating oxalic acid with phosphorus pentachloride. Oxalyl chloride is produced commercially from ethylene carbonate. Photochlorination gives the tetrachloride, which is subsequently degraded: :C2H4O2CO + 4 Cl2 → C2Cl4O2CO + 4 HCl :C2Cl4O2CO → C2O2Cl2 + COCl2 Reactions Oxalyl chloride reacts with water giving off gaseous products only: hydrogen chloride (HCl), carbon dioxide (CO2), and carbon monoxide (CO). : In this, it is quite different from other acyl chlorides which hydrolyze with formation of hydrogen chloride and the original carboxylic acid. Applications in organic synthesis Oxidation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine Oxide
Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = C6H5). This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallizing of chemical compounds. Structure and properties Ph3PO is a tetrahedral molecule related to POCl3. The oxygen center is relatively basic. The rigidity of the backbone and the basicity of the oxygen center make this species a popular agent to crystallize otherwise difficult to crystallize molecules. This trick is applicable to molecules that have acidic hydrogen atoms, e.g. phenols. Up to now, several modifications of Ph3PO have been found: For example, a monoclinic form crystalizes in the space group ''P''21/''c'' with Z = 4 and a = 15.066(1) Å, b = 9.037(2) Å, c = 11.296(3) Å, and β = 98.47(1)°. The orthorhombic modification crystallizes in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

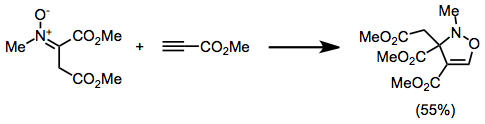




nickel(II)-from-xtal-3D-balls.png)