Suzuki coupling on:
[Wikipedia]
[Google]
[Amazon]
The Suzuki reaction or Suzuki coupling is an
 The Suzuki coupling takes place in the presence of a base and for a long time the role of the base was not fully understood. The base was first believed to form a trialkyl borate (R3B-OR), in the case of a reaction of a trialkylborane (BR3) and
The Suzuki coupling takes place in the presence of a base and for a long time the role of the base was not fully understood. The base was first believed to form a trialkyl borate (R3B-OR), in the case of a reaction of a trialkylborane (BR3) and
 Oxidative addition proceeds with retention of
Oxidative addition proceeds with retention of 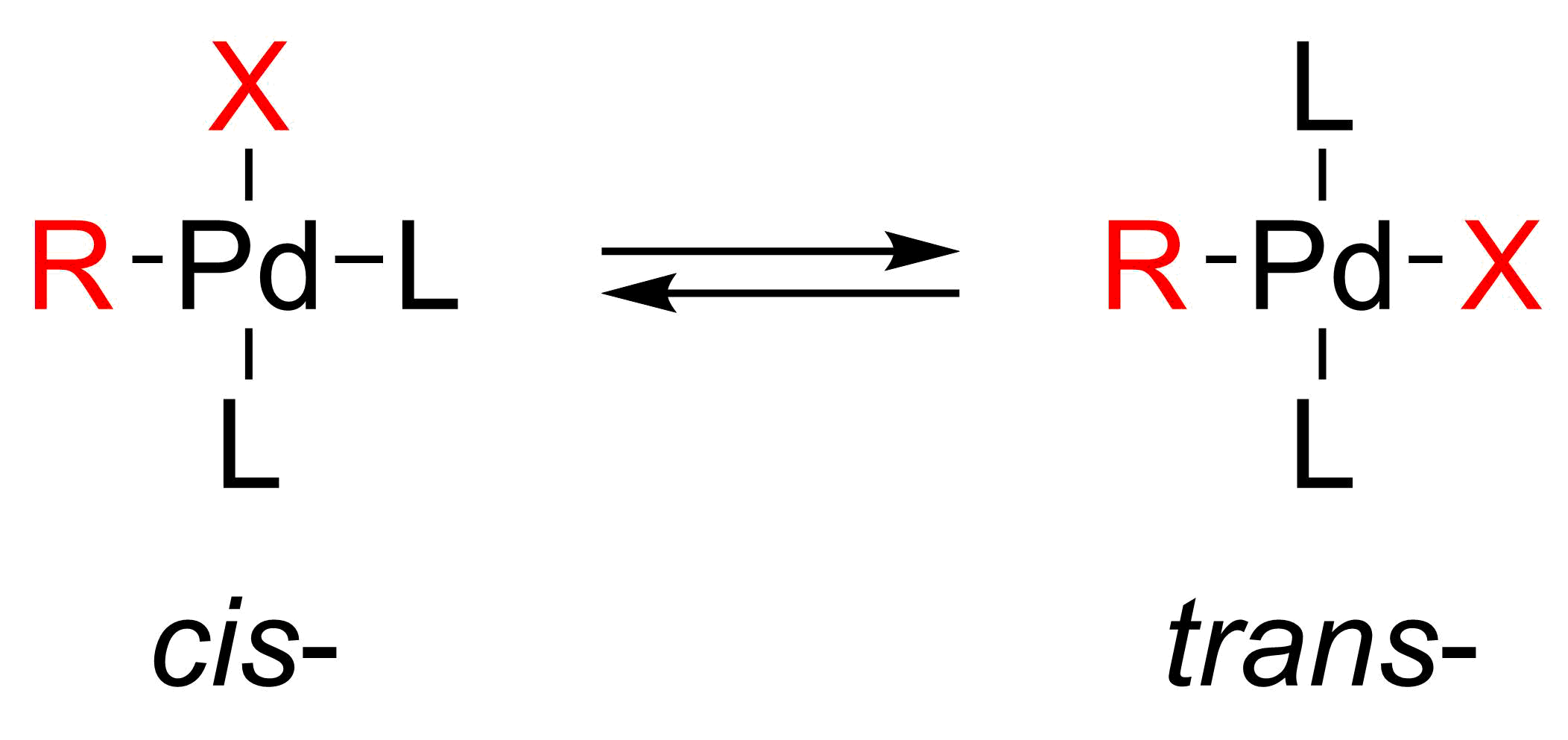 The Suzuki coupling occurs with retention of configuration on the double bonds for both the organoboron reagent or the halide. However, the configuration of that double bond, ''cis'' or ''trans'' is determined by the ''cis''-to-''trans'' isomerization of the palladium complex in the oxidative addition step where the trans palladium complex is the predominant form. When the organoboron is attached to a double bond and it is coupled to an alkenyl halide the product is a diene as shown below.
The Suzuki coupling occurs with retention of configuration on the double bonds for both the organoboron reagent or the halide. However, the configuration of that double bond, ''cis'' or ''trans'' is determined by the ''cis''-to-''trans'' isomerization of the palladium complex in the oxidative addition step where the trans palladium complex is the predominant form. When the organoboron is attached to a double bond and it is coupled to an alkenyl halide the product is a diene as shown below.


 The ligand plays an important role in the Suzuki reaction. Typically, the phosphine ligand is used in the Suzuki reaction. Phosphine ligand increases the electron density at the metal center of the complex and therefore helps in the oxidative addition step. In addition, the bulkiness of substitution of the phosphine ligand helps in the reductive elimination step. However, ''N''-heterocyclic carbene ligands have recently been used in this cross coupling, due to the instability of the phosphine ligand under Suzuki reaction conditions. ''N''-Heterocyclic carbenes are more electron rich and bulky than the phosphine ligand. Therefore, both the steric and electronic factors of the ''N''-heterocyclic carbene ligand help to stabilize active Pd(0) catalyst.
The ligand plays an important role in the Suzuki reaction. Typically, the phosphine ligand is used in the Suzuki reaction. Phosphine ligand increases the electron density at the metal center of the complex and therefore helps in the oxidative addition step. In addition, the bulkiness of substitution of the phosphine ligand helps in the reductive elimination step. However, ''N''-heterocyclic carbene ligands have recently been used in this cross coupling, due to the instability of the phosphine ligand under Suzuki reaction conditions. ''N''-Heterocyclic carbenes are more electron rich and bulky than the phosphine ligand. Therefore, both the steric and electronic factors of the ''N''-heterocyclic carbene ligand help to stabilize active Pd(0) catalyst.
 Significant efforts have been put into the development of heterogeneous catalysts for the Suzuki CC reaction, motivated by the performance gains in the industrial process (eliminating the catalyst separation from the substrate), and recently a Pd single atom heterogeneous catalyst has been shown to outperform the industry default homogeneous Pd(PPh3)4 catalyst.
Significant efforts have been put into the development of heterogeneous catalysts for the Suzuki CC reaction, motivated by the performance gains in the industrial process (eliminating the catalyst separation from the substrate), and recently a Pd single atom heterogeneous catalyst has been shown to outperform the industry default homogeneous Pd(PPh3)4 catalyst.
 Investigation into the nickel catalyzed cross-coupling continued and increased the scope of the reaction after these first examples were shown and the research interest grew. Miyaura and Inada reported in 2000 that a cheaper nickel catalyst could be utilized for the cross-coupling, using triphenylphosphine (PPh3) instead of the more expensive
Investigation into the nickel catalyzed cross-coupling continued and increased the scope of the reaction after these first examples were shown and the research interest grew. Miyaura and Inada reported in 2000 that a cheaper nickel catalyst could be utilized for the cross-coupling, using triphenylphosphine (PPh3) instead of the more expensive 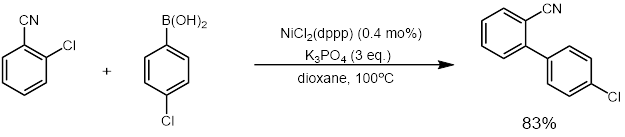 It was also reported by Wu and co-workers in 2011 that a highly active nickel catalyst for the cross-coupling of aryl chlorides could be used that only required 0.01-0.1 mol% of nickel catalyst. They also showed that the catalyst could be recycled up to six times with virtually no loss in catalytic activity. The catalyst was recyclable because it was a phosphine nickel
It was also reported by Wu and co-workers in 2011 that a highly active nickel catalyst for the cross-coupling of aryl chlorides could be used that only required 0.01-0.1 mol% of nickel catalyst. They also showed that the catalyst could be recycled up to six times with virtually no loss in catalytic activity. The catalyst was recyclable because it was a phosphine nickel  Advantages and disadvantages apply to both the palladium and nickel-catalyzed Suzuki coupling reactions. Apart from Pd and Ni catalyst system, cheap and non-toxic metal sources like iron and copper have been used in Suzuki coupling reaction. The Bedford research group and the Nakamura research group have extensively worked on developing the methodology of iron catalyzed Suzuki coupling reaction. Ruthenium is another metal source that has been used in Suzuki coupling reaction.
Advantages and disadvantages apply to both the palladium and nickel-catalyzed Suzuki coupling reactions. Apart from Pd and Ni catalyst system, cheap and non-toxic metal sources like iron and copper have been used in Suzuki coupling reaction. The Bedford research group and the Nakamura research group have extensively worked on developing the methodology of iron catalyzed Suzuki coupling reaction. Ruthenium is another metal source that has been used in Suzuki coupling reaction.
 The synthesis of a
The synthesis of a 
Suzuki coupling
{{Authority control Carbon-carbon bond forming reactions Palladium Substitution reactions Name reactions
organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
that uses a palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki
is a Japanese chemist and Nobel Prize Laureate (2010), who first published the Suzuki reaction, the organic reaction of an aryl- or vinyl- boronic acid with an aryl- or vinyl- halide catalyzed by a palladium(0) complex, in 1979.
Early life a ...
, and he shared the 2010 Nobel Prize in Chemistry
The Nobel Prize in Chemistry () is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outst ...
with Richard F. Heck and Ei-ichi Negishi
was a Japanese chemist who was best known for his discovery of the Negishi coupling. He spent most of his career at Purdue University in the United States, where he was the Herbert C. Brown Distinguished Professor and the director of the Negi ...
for their contribution to the discovery and development of noble metal catalysis in organic synthesis. This reaction is sometimes telescoped with the related Miyaura borylation; the combination is the Suzuki–Miyaura reaction. It is widely used to synthesize polyolefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
s, styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
s, and substituted biphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
s.
The general scheme for the Suzuki reaction is shown below, where a carbon–carbon single bond is formed by coupling a halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
(R1-X) with an organoboron species (R2-BY2) using a palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
catalyst and a base. The organoboron species is usually synthesized by hydroboration or carboboration, allowing for rapid generation of molecular complexity.
Several reviews have been published describing advancements and the development of the Suzuki reaction.
Reaction mechanism
Themechanism
Mechanism may refer to:
*Mechanism (economics), a set of rules for a game designed to achieve a certain outcome
**Mechanism design, the study of such mechanisms
*Mechanism (engineering), rigid bodies connected by joints in order to accomplish a ...
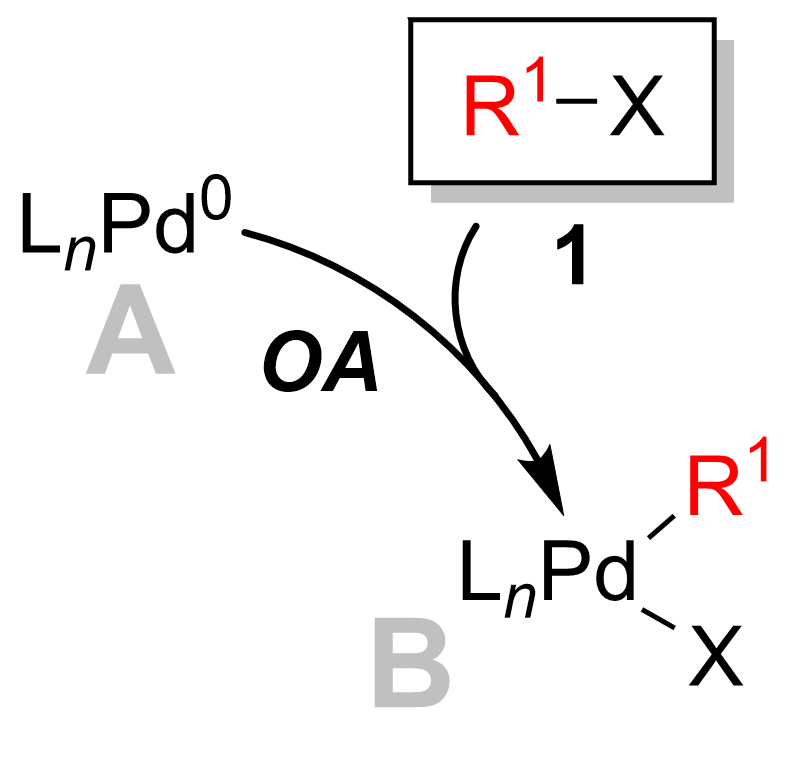
of the Suzuki reaction is best viewed from the perspective of the palladium catalyst. The catalytic cycle is initiated by the formation of an active Pd0 catalytic species, A. This participates in the oxidative addition of palladium to the halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
reagent 1 to form the organopalladium intermediate B. Reaction ( metathesis) with base gives intermediate C, which via transmetalation with the boron- ate complex D (produced by reaction of the boronic acid reagent 2 with base) forms the transient organopalladium species E. Reductive elimination step leads to the formation of the desired product 3 and restores the original palladium catalyst A which completes the catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
.
 The Suzuki coupling takes place in the presence of a base and for a long time the role of the base was not fully understood. The base was first believed to form a trialkyl borate (R3B-OR), in the case of a reaction of a trialkylborane (BR3) and
The Suzuki coupling takes place in the presence of a base and for a long time the role of the base was not fully understood. The base was first believed to form a trialkyl borate (R3B-OR), in the case of a reaction of a trialkylborane (BR3) and alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, whe ...
(−OR); this species could be considered as being more nucleophilic and then more reactive towards the palladium complex present in the transmetalation step. Duc and coworkers investigated the role of the base in the reaction mechanism for the Suzuki coupling and they found that the base has three roles: Formation of the palladium complex rPd(OR)L2 formation of the trialkyl borate and the acceleration of the reductive elimination step by reaction of the alkoxide with the palladium complex.
Oxidative addition
In most cases the oxidative addition is therate determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
of the catalytic cycle. During this step, the palladium catalyst is oxidized
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
from palladium(0) to palladium(II). The catalytically active palladium species A is coupled with the aryl halide substrate 1 to yield an organopalladium complex B. As seen in the diagram below, the oxidative addition step breaks the carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
-halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
bond where the palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
is now bound to both the halogen (X) as well as the R1 group.
 Oxidative addition proceeds with retention of
Oxidative addition proceeds with retention of stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
with vinyl halides, while giving inversion of stereochemistry with allylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolat ...
and benzylic halides. The oxidative addition initially forms the cis–palladium complex, which rapidly isomerizes to the trans-complex.
 The Suzuki coupling occurs with retention of configuration on the double bonds for both the organoboron reagent or the halide. However, the configuration of that double bond, ''cis'' or ''trans'' is determined by the ''cis''-to-''trans'' isomerization of the palladium complex in the oxidative addition step where the trans palladium complex is the predominant form. When the organoboron is attached to a double bond and it is coupled to an alkenyl halide the product is a diene as shown below.
The Suzuki coupling occurs with retention of configuration on the double bonds for both the organoboron reagent or the halide. However, the configuration of that double bond, ''cis'' or ''trans'' is determined by the ''cis''-to-''trans'' isomerization of the palladium complex in the oxidative addition step where the trans palladium complex is the predominant form. When the organoboron is attached to a double bond and it is coupled to an alkenyl halide the product is a diene as shown below.

Transmetalation
Transmetalation is anorganometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
reaction where ligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ...
are transferred from one species to another. In the case of the Suzuki coupling the ligands are transferred from the organoboron species D to the palladium(II) complex C where the base that was added in the prior step is exchanged with the R2 substituent on the organoboron species to give the new palladium(II) complex E. The exact mechanism of transmetalation for the Suzuki coupling remains to be discovered. The organoboron compounds do not undergo transmetalation in the absence of base and it is therefore widely believed that the role of the base is to activate the organoboron compound as well as facilitate the formation of R1-Pdll-O''t''Bu intermediate (C) from oxidative addition product R1-Pdll-X (B).

Reductive elimination
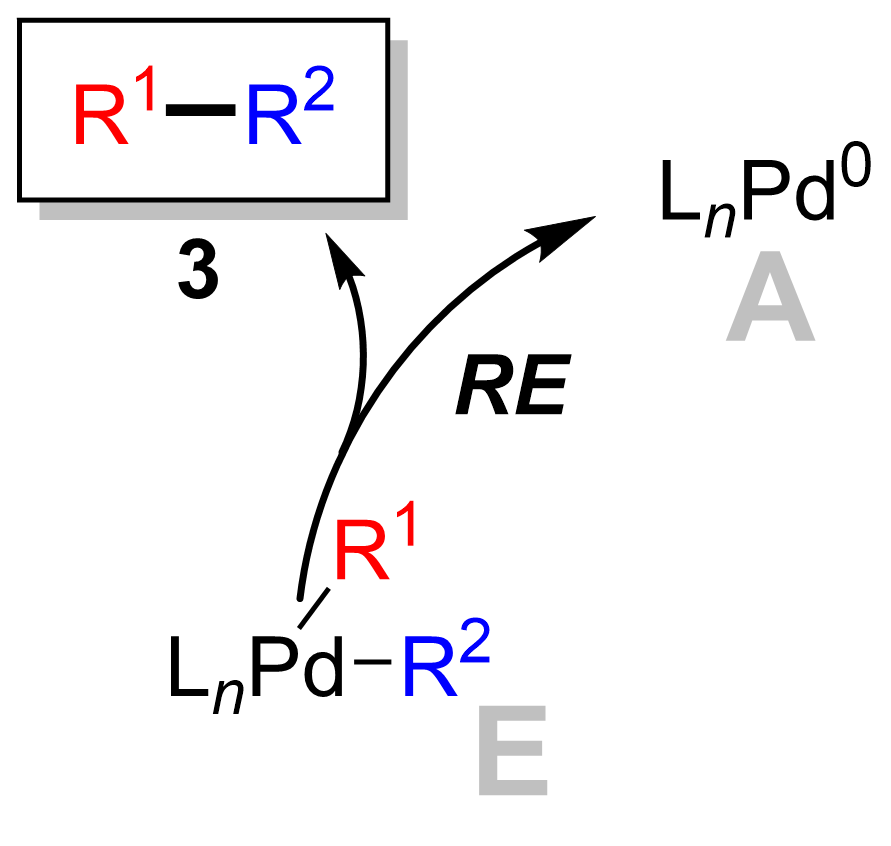
The final step is the reductive elimination step where the palladium(II) complex (E) eliminates the product (3) and regenerates the palladium(0) catalyst (A). Using deuterium labelling, Ridgway ''et al.'' have shown the reductive elimination proceeds with retention of stereochemistry. The ligand plays an important role in the Suzuki reaction. Typically, the phosphine ligand is used in the Suzuki reaction. Phosphine ligand increases the electron density at the metal center of the complex and therefore helps in the oxidative addition step. In addition, the bulkiness of substitution of the phosphine ligand helps in the reductive elimination step. However, ''N''-heterocyclic carbene ligands have recently been used in this cross coupling, due to the instability of the phosphine ligand under Suzuki reaction conditions. ''N''-Heterocyclic carbenes are more electron rich and bulky than the phosphine ligand. Therefore, both the steric and electronic factors of the ''N''-heterocyclic carbene ligand help to stabilize active Pd(0) catalyst.
The ligand plays an important role in the Suzuki reaction. Typically, the phosphine ligand is used in the Suzuki reaction. Phosphine ligand increases the electron density at the metal center of the complex and therefore helps in the oxidative addition step. In addition, the bulkiness of substitution of the phosphine ligand helps in the reductive elimination step. However, ''N''-heterocyclic carbene ligands have recently been used in this cross coupling, due to the instability of the phosphine ligand under Suzuki reaction conditions. ''N''-Heterocyclic carbenes are more electron rich and bulky than the phosphine ligand. Therefore, both the steric and electronic factors of the ''N''-heterocyclic carbene ligand help to stabilize active Pd(0) catalyst.
Advantages
The advantages of Suzuki coupling over other similar reactions include availability of common boronic acids, mild reaction conditions, and its less toxic nature. Boronic acids are less toxic and safer for the environment thanorganotin
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discove ...
and organozinc compound
Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.The Chemistry of Organozinc Compounds' (Patai S ...
s. It is easy to remove the inorganic by-products from the reaction mixture. Further, this reaction is preferable because it uses relatively cheap and easily prepared reagents. Being able to use water as a solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
makes this reaction more economical, eco-friendly, and practical to use with a variety of water-soluble reagents. A wide variety of reagents can be used for the Suzuki coupling, e.g., aryl or vinyl boronic acids and aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
or vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
halides. Work has also extended the scope of the reaction to incorporate alkyl bromides. In addition to many different type of halides being possible for the Suzuki coupling reaction, the reaction also works with pseudohalides such as triflate
In organic chemistry, triflate (Preferred IUPAC name, systematic name: trifluoromethanesulfonate), is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflate group is often represented by , as opp ...
s (OTf), as replacements for halides
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluo ...
. The relative reactivity for the coupling partner with the halide or pseudohalide is: R2–I > R2–OTf > R2–Br ≫ R2–Cl. Boronic esters and organotrifluoroborate salts may be used instead of boronic acids. The catalyst can also be a palladium nanomaterial-based catalyst. With a novel organophosphine ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
( SPhos), a catalyst loading of down to 0.001 mol% has been reported. These advances and the overall flexibility of the process have made the Suzuki coupling widely accepted for chemical synthesis.
Applications
Industrial applications
The Suzuki coupling reaction is scalable and cost-effective for use in the synthesis of intermediates forpharmaceuticals
Medication (also called medicament, medicine, pharmaceutical drug, medicinal product, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the ...
or fine chemicals
In chemistry, fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used f ...
. The Suzuki reaction was once limited by high levels of catalyst and the limited availability of boronic acids. Replacements for halides were also found, increasing the number of coupling partners for the halide or pseudohalide as well. Scaled up reactions have been carried out in the synthesis of a number of important biological compounds such as CI-1034 which used triflate
In organic chemistry, triflate (Preferred IUPAC name, systematic name: trifluoromethanesulfonate), is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflate group is often represented by , as opp ...
and boronic acid coupling partners which was run on an 80 kilogram scale with a 95% yield.
Another example is the coupling of 3-pyridylborane and 1-bromo-3-(methylsulfonyl)benzene that formed an intermediate that was used in the synthesis of a potential central nervous system agent. The coupling reaction to form the intermediate produced 278 kilograms in a 92.5% yield.
 Significant efforts have been put into the development of heterogeneous catalysts for the Suzuki CC reaction, motivated by the performance gains in the industrial process (eliminating the catalyst separation from the substrate), and recently a Pd single atom heterogeneous catalyst has been shown to outperform the industry default homogeneous Pd(PPh3)4 catalyst.
Significant efforts have been put into the development of heterogeneous catalysts for the Suzuki CC reaction, motivated by the performance gains in the industrial process (eliminating the catalyst separation from the substrate), and recently a Pd single atom heterogeneous catalyst has been shown to outperform the industry default homogeneous Pd(PPh3)4 catalyst.
Synthetic applications
The Suzuki coupling has been frequently used in syntheses of complex compounds. The Suzuki coupling has been used on a citronellal derivative for the synthesis of caparratriene, a natural product that is highly active against leukemia:Variations
Metal catalyst
Various catalytic uses of metals other than palladium (especially nickel) have been developed. The first nickel catalyzed cross-coupling reaction was reported by Percec and co-workers in 1995 using aryl mesylates and boronic acids. Even though a higher amount of nickelcatalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
was needed for the reaction, around 5 mol %, nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
is not as expensive or as precious a metal as palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
. The nickel catalyzed Suzuki coupling reaction also allowed a number of compounds that did not work or worked worse for the palladium catalyzed system than the nickel-catalyzed system. The use of nickel catalysts has allowed for electrophiles that proved challenging for the original Suzuki coupling using palladium, including substrates such as phenols, aryl ethers, esters, phosphates, and fluorides.
 Investigation into the nickel catalyzed cross-coupling continued and increased the scope of the reaction after these first examples were shown and the research interest grew. Miyaura and Inada reported in 2000 that a cheaper nickel catalyst could be utilized for the cross-coupling, using triphenylphosphine (PPh3) instead of the more expensive
Investigation into the nickel catalyzed cross-coupling continued and increased the scope of the reaction after these first examples were shown and the research interest grew. Miyaura and Inada reported in 2000 that a cheaper nickel catalyst could be utilized for the cross-coupling, using triphenylphosphine (PPh3) instead of the more expensive ligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ...
previously used. However, the nickel-catalyzed cross-coupling still required high catalyst loadings (3-10%), required excess ligand (1-5 equivalents) and remained sensitive to air and moisture. Advancements by Han and co-workers have tried to address that problem by developing a method using low amounts of nickel catalyst (<1 mol%) and no additional equivalents of ligand.
 It was also reported by Wu and co-workers in 2011 that a highly active nickel catalyst for the cross-coupling of aryl chlorides could be used that only required 0.01-0.1 mol% of nickel catalyst. They also showed that the catalyst could be recycled up to six times with virtually no loss in catalytic activity. The catalyst was recyclable because it was a phosphine nickel
It was also reported by Wu and co-workers in 2011 that a highly active nickel catalyst for the cross-coupling of aryl chlorides could be used that only required 0.01-0.1 mol% of nickel catalyst. They also showed that the catalyst could be recycled up to six times with virtually no loss in catalytic activity. The catalyst was recyclable because it was a phosphine nickel nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At ...
catalyst (G3DenP-Ni) that was made from dendrimer
Dendrimers are highly ordered, Branching (polymer chemistry), branched molecules, polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a sph ...
s.
 Advantages and disadvantages apply to both the palladium and nickel-catalyzed Suzuki coupling reactions. Apart from Pd and Ni catalyst system, cheap and non-toxic metal sources like iron and copper have been used in Suzuki coupling reaction. The Bedford research group and the Nakamura research group have extensively worked on developing the methodology of iron catalyzed Suzuki coupling reaction. Ruthenium is another metal source that has been used in Suzuki coupling reaction.
Advantages and disadvantages apply to both the palladium and nickel-catalyzed Suzuki coupling reactions. Apart from Pd and Ni catalyst system, cheap and non-toxic metal sources like iron and copper have been used in Suzuki coupling reaction. The Bedford research group and the Nakamura research group have extensively worked on developing the methodology of iron catalyzed Suzuki coupling reaction. Ruthenium is another metal source that has been used in Suzuki coupling reaction.
Amide coupling
Nickel catalysis can construct C-C bonds from amides. Despite the inherently inert nature of amides as synthons, the following methodology can be used to prepare C-C bonds. The coupling procedure is mild and tolerant of myriad functional groups, including: amines, ketones, heterocycles, groups with acidic protons. This technique can also be used to prepare bioactive molecules and to unite heterocycles in controlled ways through shrewd sequential cross-couplings. A general review of the reaction scheme is given below. The synthesis of a
The synthesis of a tubulin
Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytosk ...
-binding compound ( antiproliferative agent) was carried out using a trimethoxybenzamide
Benzamide is an organic compound with the chemical formula of C7H7NO. It is the simplest amide derivative of benzoic acid. In powdered form, it appears as a white solid, while in crystalline form, it appears as colourless crystals. It is slightly ...
and an indolyl pinacol
Organoboranes
Aryl boronic acids are comparatively cheaper than other organoboranes and a wide variety of aryl boronic acids are commercially available. Hence, it has been widely used in Suzuki reaction as an organoborane partner. Aryltrifluoroborate salts are another class of organoboranes that are frequently used because they are less prone to protodeboronation compared to aryl boronic acids. They are easy to synthesize and can be easily purified. Aryltrifluoroborate salts can be formed from boronic acids by the treatment with potassium hydrogen fluoride which can then be used in the Suzuki coupling reaction.Solvent variations
The Suzuki coupling reaction is different from other coupling reactions in that it can be run in biphasic organic-water, water-only, or no solvent. This increased the scope of coupling reactions, as a variety of water-soluble bases, catalyst systems, and reagents could be used without concern over their solubility in organic solvent. Use of water as a solvent system is also attractive because of the economic and safety advantages. Frequently used in solvent systems for Suzuki coupling aretoluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
, THF, dioxane, and DMF. The most frequently used bases are K2CO3, KO''t''Bu, Cs2CO3, K3PO4, NaOH, and NEt3.
See also
* Chan-Lam coupling * Heck reaction * Hiyama coupling *Kumada coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically ...
* Negishi coupling
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon–carbon bonds (C–C) in the process. A palladium (0) s ...
* Petasis reaction
* Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vi ...
* Stille reaction
* List of organic reactions
References
External links
*Suzuki coupling
{{Authority control Carbon-carbon bond forming reactions Palladium Substitution reactions Name reactions