Sulfotep on:
[Wikipedia]
[Google]
[Amazon]
Sulfotep (also known as tetraethyldithiopyrophosphate and TEDP) is a pesticide commonly used in greenhouses as a
 An alternative route to TEPP can be a reaction of diethyl chlorothiophosphate an aqueous solution of
An alternative route to TEPP can be a reaction of diethyl chlorothiophosphate an aqueous solution of
fumigant
Fumigation is a method of pest control or the removal of harmful micro-organisms by completely filling an area with gaseous pesticides—or fumigants—to suffocate or poison the pests within. It is used to control pests in buildings ( ...
. The substance is also known as Dithione, Dithiophos, and many other names. Sulfotep has the molecular formula C8H20O5P2S2 and belongs to the organophosphate
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered a ...
class of chemicals. It has a cholinergic effect, involving depression of the cholinesterase activity of the peripheral and central nervous system of insects. The transduction of signals is disturbed at the synapses that make use of acetylcholine
Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Part ...
. Sulfotep is a mobile oil that is pale yellow-colored and smells like garlic
Garlic (''Allium sativum'') is a species of bulbous flowering plant in the genus ''Allium''. Its close relatives include the onion, shallot, leek, chive, Allium fistulosum, Welsh onion and Allium chinense, Chinese onion. It is native to South A ...
. It is primarily used as an insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to b ...
.
History
Sulfotep was first commercially launched by Bayer in 1946. The first time that tetraethyl dithiopyrophosphate was registered to be used in theUnited States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territorie ...
was in 1951. A Registration Standard for the chemical was issued by the Environmental Protection Agency
A biophysical environment is a biotic and abiotic surrounding of an organism or population, and consequently includes the factors that have an influence in their survival, development, and evolution. A biophysical environment can vary in scale f ...
in September 1988. Plans were made in 1999 by the Environmental Protection Agency to stop production of it by September 30, 2002, and to outlaw the use and distribution of products containing it by September 30, 2004.
Chemistry
Synthesis
Sulfotep is synthesized by a reaction oftetraethyl pyrophosphate
Tetraethyl pyrophosphate, abbreviated TEPP, is an organophosphate compound with the formula . It is the tetraEthyl group, ethyl derivative of pyrophosphate (P2O74-). It is a colorless oil that solidifies near room temperature. It is used as an ins ...
(TEPP) with sulfur. TEPP itself was first synthesized by Philipe de Clermont in 1854. TEPP is made by a reaction of diethyl chlorophosphate
Diethyl chlorophosphate is an organophosphorus compound with the formula (C2H5O)2P(O)Cl. As an reagent in organic synthesis, it is use the convert alcohols to the corresponding diethylphosphate esters. It is a colorless liquid with a fruity odor. ...
with water to substitute the chloro group with a hydroxyl group. The product can react with another molecule of diethylchlorophosphate to form the ester, TEPP. In this reaction, pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
is often used to neutralize the hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
byproduct.
: An alternative route to TEPP can be a reaction of diethyl chlorothiophosphate an aqueous solution of
An alternative route to TEPP can be a reaction of diethyl chlorothiophosphate an aqueous solution of sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
(Na2CO3).
Properties
When heated to a temperature that is high enough for sulfotep to decompose, it gives off fumes ofphosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
and sulfur oxides, which are highly toxic. It can explode
An explosion is a rapid expansion in volume associated with an extreme outward release of energy, usually with the generation of high temperatures and release of high-pressure gases. Supersonic explosions created by high explosives are known ...
if containers of it are heated, and it can burn, although it does not do so easily. The chemical can also polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
ize explosively. The chemical also reacts to form toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
and flammable
A combustible material is something that can burn (i.e., ''combust'') in air. A combustible material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort and a flammable mat ...
gases in the presence of hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
s and other reducing agents. It is able to corrode iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
. When it does this, it can release hydrogen gas. The chemical has a specific gravity
Relative density, or specific gravity, is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water (molecule), wa ...
of 1.196 at and its vapor density is 13.17 grams per liter at . Its melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends ...
is and its boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
is between and at 2 mmHg. The chemical's sorption coefficient
Sorption is a physical and chemical process by which one substance becomes attached to another. Specific cases of sorption are treated in the following articles:
; Absorption: "the incorporation of a substance in one state into another of a diff ...
is 2.87 Log L/kg. Its Henry's Law constant is 0.000175 at . Its octanol-water partition coefficient is 3.9804 Log L/kg. Tetraethyl dithiopyrophosphate's diffusion coefficient in air is 0.015 cm2 per second and its diffusion coefficient in water is 0.0000055 cm2.
Sulfotep's flash point
The flash point of a material is the "lowest liquid temperature at which, under certain standardized conditions, a liquid gives off vapours in a quantity such as to be capable of forming an ignitable vapour/air mixture". (EN 60079-10-1)
The fl ...
is and its enthalpy of vaporization is 59.4 kilojoules per mole
Mole (or Molé) may refer to:
Animals
* Mole (animal) or "true mole", mammals in the family Talpidae, found in Eurasia and North America
* Golden moles, southern African mammals in the family Chrysochloridae, similar to but unrelated to Talpida ...
. Its surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to f ...
is 423 dynes per centimeter. The chemical has no Rule of 5 violations. Its diffusivity
Diffusivity is a rate of diffusion, a measure of the rate at which particles or heat or fluids can spread.
It is measured differently for different mediums.
Diffusivity may refer to:
*Thermal diffusivity, diffusivity of heat
*Diffusivity of mass: ...
in water is 0.63 × 10−5 cm2 per second. It is miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). The term is most often applied to liquids but also applies ...
with a large number of organic solvents, including methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industrial ...
and acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
and its solubility in water is 30 milligrams per liter at .
The alkaline and neutral hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of sulfotep results in the release of ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
, phosphoric acid, and hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
.
Applications
Sulfotep has applications as aninsecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to b ...
, miticide, and acaricide. However, because it does not leave behind a residue, it is less effective at these roles than DDT
Dichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound, an organochloride. Originally developed as an insecticide, it became infamous for its environmental impacts. ...
. However, it is about as effective as the insecticide parathion
Parathion, also called parathion-ethyl or diethyl parathion and locally known as "Folidol", is an organophosphate insecticide and acaricide. It was originally developed by IG Farben in the 1940s. It is highly toxic to non-target organisms, incl ...
. Its use is restricted to greenhouse
A greenhouse (also called a glasshouse, or, if with sufficient heating, a hothouse) is a structure with walls and roof made chiefly of Transparent ceramics, transparent material, such as glass, in which plants requiring regulated climatic condit ...
s and ornamental plant
Ornamental plants or garden plants are plants that are primarily grown for their beauty but also for qualities such as scent or how they shape physical space. Many flowering plants and garden varieties tend to be specially bred cultivars that i ...
s. When the chemical is used as an insecticide, it is in the form of an impregnated smoke fumigant. Sulfotep is used in greenhouses as a fumigant formulation to control aphids, spider mites, whiteflies and thrips. It is formulated as impregnated material in smoke generators containing 14 to 15% active ingredient. Smoke generators are placed in the greenhouses and then ignited using inserted sparklers to generate a thick white smoke for fumigation.
Sulfotep kills spider mite
Spider mites are members of the Tetranychidae family, which includes about 1,200 species. They are part of the subclass Acari (mites). Spider mites generally live on the undersides of leaves of plants, where they may spin protective silk webs, a ...
s, mealybug
Mealybugs are insects in the family (biology), family Pseudococcidae, unarmored scale insects found in moist, warm habitats. Many species are considered pest (animal), pests as they feed on plant juices of greenhouse plants, house plants and sub ...
s, whiteflies
Whiteflies are Hemipterans that typically feed on the undersides of plant leaves. They comprise the family Aleyrodidae, the only family in the superfamily Aleyrodoidea. More than 1550 species have been described.
Description and taxonomy
The ...
, and aphids. However, the chemical is not phytotoxic, unlike tetraethyl pyrophosphate
Tetraethyl pyrophosphate, abbreviated TEPP, is an organophosphate compound with the formula . It is the tetraEthyl group, ethyl derivative of pyrophosphate (P2O74-). It is a colorless oil that solidifies near room temperature. It is used as an ins ...
. However, it occasionally causes minor damage to plants, such as the slight puckering and cupping of leaves. During several tests in the late 1940s, it was found to be the most toxic of several chemicals to whiteflies on vegetable
Vegetables are parts of plants that are consumed by humans or other animals as food. The original meaning is still commonly used and is applied to plants collectively to refer to all edible plant matter, including the flowers, fruits, stems, ...
s, two-spotted spider mites on rose
A rose is either a woody perennial flowering plant of the genus ''Rosa'' (), in the family Rosaceae (), or the flower it bears. There are over three hundred species and tens of thousands of cultivars. They form a group of plants that can be ...
s, and mealybugs on numerous plants.
A mixture containing 5% sulfotep at the concentration of 0.5 grams of phosphate per 1000 cubic feet was found in tests in the late 1940s to kill 100% of nonresistant two-spotted spider mites and 68-97% of resistant two-spotted spider mites. Sulfotep aerosols killed 100% of the populations of a large number of insects, but only killed 98% of mealybugs in the same tests. 88% of nonresistant spider mites can be killed be two minutes of exposure to a mixture containing 5% of the chemical, 98-99% can be killed after five to ten minutes, and all can be killed after 15 minutes.
Mechanism of action
Sulfotep, just as allorganophosphate
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered a ...
pesticides, irreversibly inactivates acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme
Enzymes () are proteins that a ...
, which is essential to nerve function in insects, humans, and many other animals. Acetylcholinesterase normally hydrolyses acetylcholine after it was released in the synapse. When the acetylcholine is not degraded, it accumulates in the synaptic cleft. Thus, it keeps on stimulating the nerve.Sulfotep, Bayer MAK 24, Lieferung 1997
Metabolism

Uptake
Sulfotep is taken up well both orally, dermally as well as through inhalation. A few different organizations determined a maximum concentration sulfotep in the air. The maximum allowed concentration is 0.2 mg/m3.Phase I
Sulfotep is desulfurated by eithercytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are ...
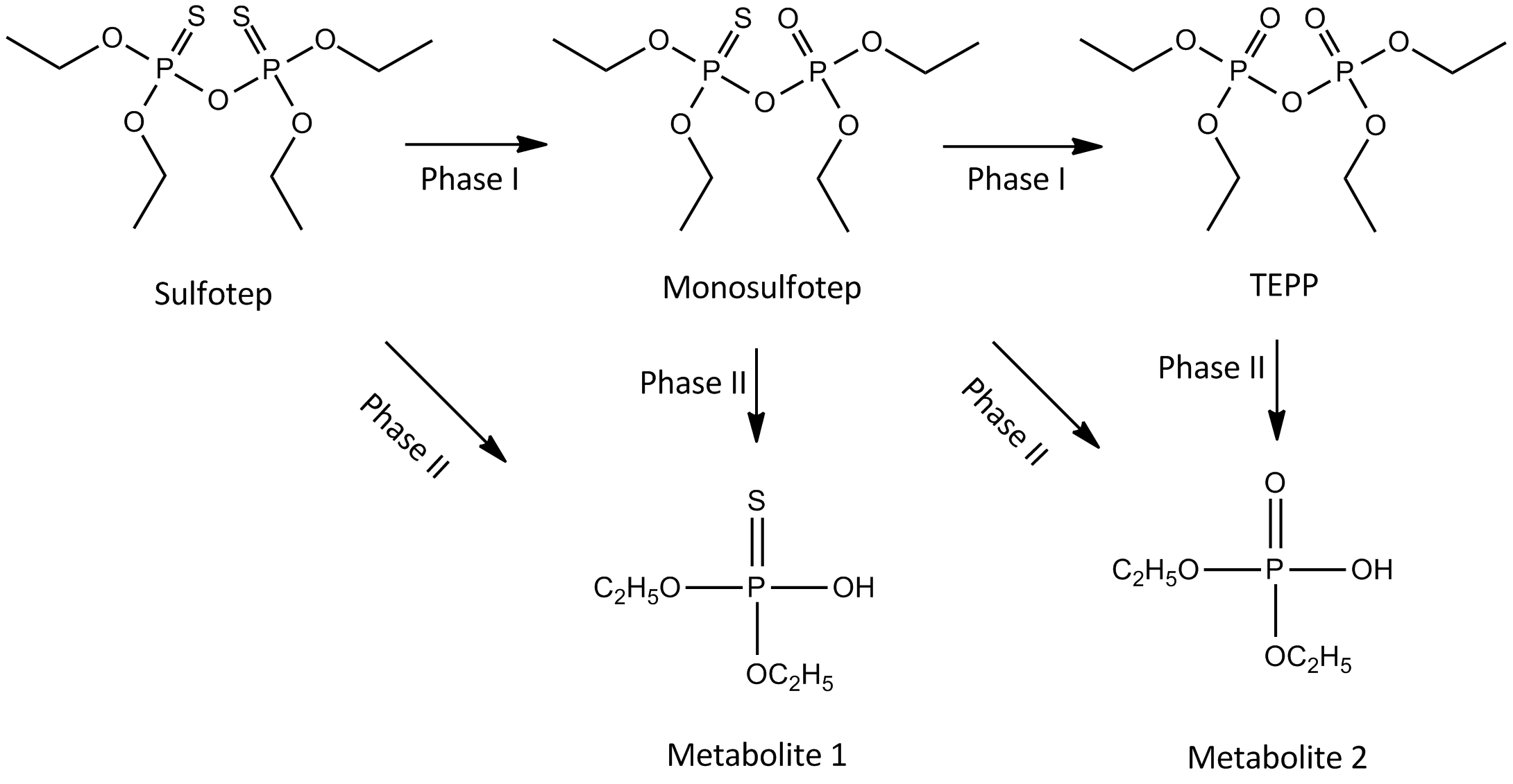
or the FAD-containing monooxygenases. In this reaction, the sulfur is replaced by oxygen, as seen in figure 2. The metabolites formed are monosulfotep and tetraethyl pyrophosphate (TEPP). To accomplish these reactions, a phospho-oxithirane ring is formed, which is highly reactive. This ring is thought to bind to acetylcholinesterase and cause toxicity.Timbrell John A., Principles of biochemical toxicology 4th edition 2009. Informa Healthcare New York. pp 91 & 99
Phase II
The two Phase I metabolites are further transformed through a hydrolysis-reaction mediated by a type A-esterase. The products formed are ''O'',''O''-diethyldithiophosphate and ''O'',''O''-diethylphosphate.Excretion
An experiment in rats who were once given 0.4 mg radioactive phosphor-labelled sulfotep orally, has shown that sulfotep is excreted by both the kidneys (urine) and the liver (bile). The substance is completely metabolised. Two metabolites are found in the urine and faeces. The radioactivity showed that 85-91% was excreted in urine and 5-6% in the faeces. * 88-96% metabolite 1: ''O'',''O''-diethyldithiophosphate * 4-12% metabolite 2: ''O'',''O''-diethylphosphateToxicity
Acute toxic effects on animals
Sulfotep is toxic to some wildlife, includingfish
Fish are aquatic, craniate, gill-bearing animals that lack limbs with digits. Included in this definition are the living hagfish, lampreys, and cartilaginous and bony fish as well as various extinct related groups. Approximately 95% of li ...
and aquatic invertebrate
Marine invertebrates are the invertebrates that live in marine habitats. Invertebrate is a blanket term that includes all animals apart from the vertebrate members of the chordate phylum. Invertebrates lack a vertebral column, and some have evo ...
s. It is also assumed by the Environmental Protection Agency to be toxic to bird
Birds are a group of warm-blooded vertebrates constituting the class Aves (), characterised by feathers, toothless beaked jaws, the laying of hard-shelled eggs, a high metabolic rate, a four-chambered heart, and a strong yet lightweigh ...
s.
Surviving animals completely recovered in 1–4 days.
Chronic and sub-chronic toxicity
A long-term exposure to a low concentration showed no toxicity. This was tested in rats. They were exposed to different concentrations of sulfotep. Exposed to the highest concentration of 2.83 mg/m3 for six hours a day, five days a week for 12 weeks, there was no change in appearance, behavior or body weight. The plasma cholinesterase activity decreased and the weight of the lungs of female rats increased. The red blood cell acetylcholinesterase activity was not affected. At lower concentrations, there were no changes at all. The rats were orally exposed to 0, 5, 10, 20 or 50 ppm sulfotep for three months. Only their plasma cholinesterase activity and RBC acetylcholinesterase activity were decreased. No further symptoms were observed. Dogs who were orally exposed to 0, 0.5, 3, 5, 15 or 75 ppm (equivalent to 0–3.07 mg/kg/day) for 13 weeks, ate less and lost weight. The plasma cholinesterase activity was already affected by a sulfotep concentration of 3 ppm (or higher). Red blood cell-acetylcholinesterase was decreased at 75 ppm. Diarrhea and vomiting occasionally occurred at 15 ppm, but were common at 75 ppm. The brain cholinesterase activity was unaffected.Poisoning symptoms and treatment
According to theOccupational Safety and Health Administration
The Occupational Safety and Health Administration'' (OSHA ) is a large regulatory agency of the United States Department of Labor that originally had federal visitorial powers to inspect and examine workplaces. Congress established the agenc ...
, the upper limit on exposure of sulfotep to human skin is 0.2 milligrams per cubic meter.
Sulfotep causes an organophosphate poisoning. This means that it had an effect on the activity of cholinesterase. There are differences for the indications of a sulfotep poisoning between inhalation, ingestion, intake by the skin and intake by the eyes. However, examples of poisoned greenhouse workers teach us an overall route of symptoms for a sulfotepp poisoning. Within the first hour after a poisonous intake of sulfotep people often suffer from nausea or headaches. After some hours diarrhea and vomiting may occur. People who inhaled sulfotep are often disorientated and have difficulties to breath. A poisonous dose may lead to a coma or death after 24 hours. The point at 24 hours after the poisoning is very important. If the dose is not lethal, the symptoms will slowly disappear after the point of 24 hours.
No embryotoxic or teratogenic effects occurred in tests. Neither were there any signs for carcinogenic effects. It was only mutagenic in one strain of ''S. typhimurium''. In four other bacterial strains as well as in rats and mice it was not mutagenic at all.
There are two cases of acute toxicity known in man. The cholinesterase activity in these people was reduced. It took them 20 respectively 28 days to recover.
The most important poisoning symptoms are shown in the following table.International Chemical Safety Cards, ICSC: 0985, https://www.cdc.gov/niosh/ipcsndut/ndut0985.html
References
External links
* {{PPDB, 604 Organophosphate insecticides Organothiophosphate esters Ethyl esters