Spiroketals on:
[Wikipedia]
[Google]
[Amazon]
In

 Due to its non-planar substructure, the spiroketal motif gain interest among the academical and industrial pharmaceutical research fields, both in structure-based drug design (SBDD) and development of screening libraries.
Due to its non-planar substructure, the spiroketal motif gain interest among the academical and industrial pharmaceutical research fields, both in structure-based drug design (SBDD) and development of screening libraries.
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, spiroketals are structural motifs composed of two heterocycles sharing one central carbon which makes them a subclass of spiro compound
In organic chemistry, spiro compounds are Organic compound, compounds that have at least two Cyclic compound, molecular rings sharing one common atom. Simple spiro compounds are bicyclic (having just two rings). The presence of only one common at ...
. Their structural specificity lays on the presence of one oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
atom in each ring, in alpha of the spiro carbon. Although there are no rules about the size of each ring, the most widely encountered spiroketal are composed of five and six membered rings.
Occurrence in nature
Many natural products of biological interest contain ,5 and ,6spiroketal moieties that can adopt various configurations. The first example of a spiroketals in the literature appeared before 1970, such as the triterpenoid saponins and sapogenins. Then several works described the presence of spiroketals in various compounds. Like diarrheic shellfish poisoning (DSP) class of toxins containing in theokadaic acid
Okadaic acid, C44H68O13, is a toxin produced by several species of dinoflagellates. It is known to accumulate in both marine sponges and shellfish. One of the primary causes of diarrhetic shellfish poisoning, okadaic acid is a potent inhibitor of ...
and ancanthafolicin. The most noticeable occurring spiroketals are the whole range of fruit fly pheromones.
Pharmacology interest
Avermectin
The avermectins are a group of 16-membered Macrolide, macrocyclic lactone derivatives with potent anthelmintic and Insecticide, insecticidal properties. These naturally occurring compounds are generated as fermentation products by ''Streptomyces a ...
s have been found in fungus and are antiparasitic drugs. The avermectins appear to paralyze nematode
The nematodes ( or ; ; ), roundworms or eelworms constitute the phylum Nematoda. Species in the phylum inhabit a broad range of environments. Most species are free-living, feeding on microorganisms, but many are parasitic. Parasitic worms (h ...
s and arthropod
Arthropods ( ) are invertebrates in the phylum Arthropoda. They possess an arthropod exoskeleton, exoskeleton with a cuticle made of chitin, often Mineralization (biology), mineralised with calcium carbonate, a body with differentiated (Metam ...
s by potentiating the presynaptic release of gamma-aminobutyric acid
GABA (gamma-aminobutyric acid, γ-aminobutyric acid) is the chief inhibitory neurotransmitter in the developmentally mature mammalian central nervous system. Its principal role is reducing neuronal excitability throughout the nervous system.
GA ...
, thereby blocking post-synaptic transmission of nerve impulses
Tofogliflozin
Tofogliflozin (INN, USAN, codenamed CSG452) is a drug developed for the treatment of diabetes mellitus type 2 and was originally co-developed by Chugai Pharma in collaboration with Kowa and Sanofi. It is an inhibitor of subtype 2 sodium-gluco ...
is an inhibitor of human sodium glucose cotransporter 2 (hSGLT2) and was approved in 2014 in Japan for the treatment of Type 2 diabetes
Chemical synthesis
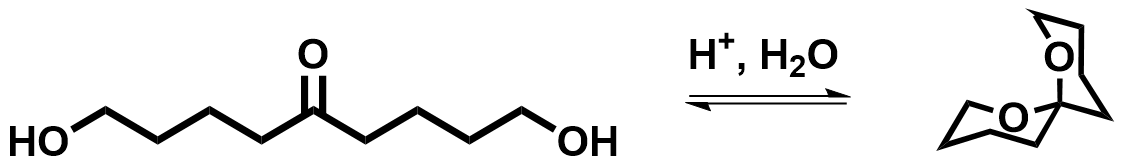
Acid catalyzed spiroketalisation
The most employed method to ring close spiroketal consists in thehydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of the dihydroxyketal in acidic conditions, but this method is not granting stereocontrol. Thus, several miscellaneous methods have emerged in order to control the stereoselectivity of the spirocyclisation.

Notes
References
{{reflist Heterocyclic compounds