Spherical Top on:
[Wikipedia]
[Google]
[Amazon]
 Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of
Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of
 The
The
 The quantum number ''J'' refers to the total angular momentum, as before. Since there are three independent moments of inertia, there are two other independent quantum numbers to consider, but the term values for an asymmetric rotor cannot be derived in closed form. They are obtained by individual
The quantum number ''J'' refers to the total angular momentum, as before. Since there are three independent moments of inertia, there are two other independent quantum numbers to consider, but the term values for an asymmetric rotor cannot be derived in closed form. They are obtained by individual
infrared gas spectra simulator
{{Branches of spectroscopy Spectroscopy Rotation Rigid bodies mechanics
 Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of
Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
s in the gas phase
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetizati ...
. The spectra of polar molecules can be measured in absorption or emission by microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequency, frequencies between 300 MHz and 300 GHz respectively. Different sources define different fre ...
spectroscopy or by far infrared
Far infrared (FIR) is a region in the infrared spectrum of electromagnetic radiation. Far infrared is often defined as any radiation with a wavelength of 15 micrometers (μm) to 1 mm (corresponding to a range of about 20 THz to ...
spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those methods, but can be observed and measured by Raman spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman s ...
. Rotational spectroscopy is sometimes referred to as ''pure'' rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy, and also from ro-vibronic spectroscopy (or just vibronic spectroscopy
Vibronic spectroscopy is a branch of molecular spectroscopy concerned with vibronic transitions: the simultaneous changes in electronic and vibrational energy levels of a molecule due to the absorption or emission of a photon of the appropriate e ...
) where rotational, vibrational and electronic energy changes occur simultaneously.
For rotational spectroscopy, molecules are classified according to symmetry into spherical top, linear and symmetric top; analytical expressions can be derived for the rotational energy terms of these molecules. Analytical expressions can be derived for the fourth category, asymmetric top, for rotational levels up to J=3, but higher energy level
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The ...
s need to be determined using numerical methods. The rotational energies are derived theoretically by considering the molecules to be rigid rotor
In rotordynamics, the rigid rotor is a mechanical model of rotating systems. An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top. To orient such an object in space requires three angles, known as Euler angles. A special ri ...
s and then applying extra terms to account for centrifugal distortion, fine structure
In atomic physics, the fine structure describes the splitting of the spectral lines of atoms due to electron spin and relativistic corrections to the non-relativistic Schrödinger equation. It was first measured precisely for the hydrogen atom b ...
, hyperfine structure
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to electromagnetic multipole interaction between the ...
and Coriolis coupling. Fitting the spectra to the theoretical expressions gives numerical values of the angular moments of inertia from which very precise values of molecular bond lengths and angles can be derived in favorable cases. In the presence of an electrostatic field there is Stark splitting which allows molecular electric dipole moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The ...
s to be determined.
An important application of rotational spectroscopy is in exploration of the chemical composition of the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstella ...
using radio telescopes.
Applications
Rotational spectroscopy has primarily been used to investigate fundamental aspects of molecular physics. It is a uniquely precise tool for the determination of molecular structure in gas phase molecules. It can be used to establish barriers to internal rotation such as that associated with the rotation of the group relative to the group inchlorotoluene Chlorotoluene is a group of three isomeric chemical compounds. They (''ortho''-chlorotoluene, ''meta''-chlorotoluene, and ''para''-chlorotoluene) consist of a disubstituted benzene ring with one chlorine atom and one methyl group.
Properties
The ...
(). When fine or hyperfine structure can be observed, the technique also provides information on the electronic structures of molecules. Much of current understanding of the nature of weak molecular interactions such as van der Waals, hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
and halogen bonds has been established through rotational spectroscopy. In connection with radio astronomy
Radio astronomy is a subfield of astronomy that studies celestial objects at radio frequencies. The first detection of radio waves from an astronomical object was in 1933, when Karl Jansky at Bell Telephone Laboratories reported radiation comi ...
, the technique has a key role in exploration of the chemical composition of the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstella ...
. Microwave transitions are measured in the laboratory and matched
to emissions from the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstella ...
using a radio telescope. was the first stable polyatomic
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may n ...
molecule to be identified in the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstella ...
. The measurement of chlorine monoxide
Chlorine monoxide is a chemical radical with the chemical formula ClO•. It plays an important role in the process of ozone depletion. In the stratosphere, chlorine atoms react with ozone molecules to form chlorine monoxide and oxygen.
:Cl� ...
is important for atmospheric chemistry
Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary approach of research and draws on environmental chemistry, physics, mete ...
. Current projects in astrochemistry involve both laboratory microwave spectroscopy and observations made using modern radiotelescopes such as the Atacama Large Millimeter/submillimeter Array (ALMA).
Overview
A molecule in thegas phase
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetizati ...
is free to rotate relative to a set of mutually orthogonal
In mathematics, orthogonality is the generalization of the geometric notion of '' perpendicularity''.
By extension, orthogonality is also used to refer to the separation of specific features of a system. The term also has specialized meanings in ...
axes of fixed orientation in space, centered on the center of mass of the molecule. Free rotation is not possible for molecules in liquid or solid phases due to the presence of intermolecular force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
s. Rotation about each unique axis is associated with a set of quantized energy levels dependent on the moment of inertia about that axis and a quantum number. Thus, for linear molecules the energy levels are described by a single moment of inertia and a single quantum number, , which defines the magnitude of the rotational angular momentum.
For nonlinear molecules which are symmetric rotors (or symmetric tops - see next section), there are two moments of inertia and the energy also depends on a second rotational quantum number, , which defines the vector component of rotational angular momentum along the principal symmetry axis. Analysis of spectroscopic data with the expressions detailed below results in quantitative determination of the value(s) of the moment(s) of inertia. From these precise values of the molecular structure and dimensions may be obtained.
For a linear molecule, analysis of the rotational spectrum provides values for the rotational constant
In rotordynamics, the rigid rotor is a mechanical model of rotating systems. An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top. To orient such an object in space requires three angles, known as Euler angles. A special ri ...
This article uses the molecular spectroscopist's convention of expressing the rotational constant in cm−1. Therefore in this article corresponds to in the Rigid rotor article. and the moment of inertia of the molecule, and, knowing the atomic masses, can be used to determine the bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest ...
directly. For diatomic molecules
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. Ot ...
this process is straightforward. For linear molecules with more than two atoms it is necessary to measure the spectra of two or more isotopologue
In chemistry, isotopologues are molecules that differ only in their isotopic composition. They have the same chemical formula and bonding arrangement of atoms, but at least one atom has a different number of neutrons than the parent.
An examp ...
s, such as 16O12C32S and 16O12C34S. This allows a set of simultaneous equations
In mathematics, a set of simultaneous equations, also known as a system of equations or an equation system, is a finite set of equations for which common solutions are sought. An equation system is usually classified in the same manner as single ...
to be set up and solved for the bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest ...
s).For a symmetric top, the values of the 2 moments of inertia can be used to derive 2 molecular parameters. Values from each additional isotopologue provide the information for one more molecular parameter. For asymmetric tops a single isotopologue provides information for at most 3 molecular parameters. A bond length obtained in this way is slightly different from the equilibrium bond length. This is because there is zero-point energy
Zero-point energy (ZPE) is the lowest possible energy that a quantum mechanical system may have. Unlike in classical mechanics, quantum systems constantly Quantum fluctuation, fluctuate in their lowest energy state as described by the Heisen ...
in the vibrational ground state, to which the rotational states refer, whereas the equilibrium bond length is at the minimum in the potential energy curve. The relation between the rotational constants is given by
:
where v is a vibrational quantum number and α is a vibration-rotation interaction constant which can be calculated if the B values for two different vibrational states can be found.
For other molecules, if the spectra can be resolved and individual transitions assigned both bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest ...
s and bond angles
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determ ...
can be deduced. When this is not possible, as with most asymmetric tops, all that can be done is to fit the spectra to three moments of inertia calculated from an assumed molecular structure. By varying the molecular structure the fit can be improved, giving a qualitative estimate of the structure. Isotopic substitution is invaluable when using this approach to the determination of molecular structure.
Classification of molecular rotors
Inquantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, q ...
the free rotation of a molecule is quantized, so that the rotational energy
Rotational energy or angular kinetic energy is kinetic energy due to the rotation of an object and is part of its total kinetic energy. Looking at rotational energy separately around an object's axis of rotation, the following dependence on the o ...
and the angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed sy ...
can take only certain fixed values, which are related simply to the moment of inertia
The moment of inertia, otherwise known as the mass moment of inertia, angular mass, second moment of mass, or most accurately, rotational inertia, of a rigid body is a quantity that determines the torque needed for a desired angular accele ...
, , of the molecule. For any molecule, there are three moments of inertia: , and about three mutually orthogonal axes ''A'', ''B'', and ''C'' with the origin at the center of mass of the system. The general convention, used in this article, is to define the axes such that , with axis corresponding to the smallest moment of inertia. Some authors, however, define the axis as the molecular rotation axis
Rotation around a fixed axis is a special case of rotational motion. The fixed- axis hypothesis excludes the possibility of an axis changing its orientation and cannot describe such phenomena as wobbling or precession. According to Euler's r ...
of highest order.
The particular pattern of energy level
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The ...
s (and, hence, of transitions in the rotational spectrum) for a molecule is determined by its symmetry. A convenient way to look at the molecules is to divide them into four different classes, based on the symmetry of their structure. These are
Selection rules
Microwave and far-infrared spectra
Transitions between rotational states can be observed in molecules with a permanentelectric dipole moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The ...
.Such transitions are called electric dipole-allowed transitions. Other transitions involving quadrupoles, octupoles, hexadecapoles etc. may also be allowed but the spectral intensity is very much smaller, so these transitions are difficult to observe. Magnetic-dipole-allowed transitions can occur in paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
molecules such as dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are: ...
, and nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its ...
, NO A consequence of this rule is that no microwave spectrum can be observed for centrosymmetric linear molecules such as ( dinitrogen) or HCCH (ethyne
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
), which are non-polar. Tetrahedral molecules such as (methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ear ...
), which have both a zero dipole moment and isotropic polarizability, would not have a pure rotation spectrum but for the effect of centrifugal distortion; when the molecule rotates about a 3-fold symmetry axis a small dipole moment is created, allowing a weak rotation spectrum to be observed by microwave spectroscopy.
With symmetric tops, the selection rule for electric-dipole-allowed pure rotation transitions is , . Since these transitions are due to absorption (or emission) of a single photon with a spin of one, conservation of angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed syste ...
implies that the molecular angular momentum can change by at most one unit. Moreover, the quantum number ''K'' is limited to have values between and including +''J'' to -''J''.
Raman spectra
For Raman spectra the molecules undergo transitions in which an ''incident'' photon is absorbed and another ''scattered'' photon is emitted. The general selection rule for such a transition to be allowed is that the molecularpolarizability
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of all matter, considering that matter is made up of elementar ...
must be anisotropic
Anisotropy () is the property of a material which allows it to change or assume different properties in different directions, as opposed to isotropy. It can be defined as a difference, when measured along different axes, in a material's phys ...
, which means that it is not the same in all directions. Polarizability is a 3-dimensional tensor
In mathematics, a tensor is an algebraic object that describes a multilinear relationship between sets of algebraic objects related to a vector space. Tensors may map between different objects such as vectors, scalars, and even other tens ...
that can be represented as an ellipsoid. The polarizability ellipsoid of spherical top molecules is in fact spherical so those molecules show no rotational Raman spectrum. For all other molecules both Stokes and anti-Stokes linesIn Raman spectroscopy the photon energies for Stokes and anti-Stokes scattering are respectively less than and greater than the incident photon energy. See the energy-level diagram at Raman spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman s ...
. can be observed and they have similar intensities due to the fact that many rotational states are thermally populated. The selection rule for linear molecules is ΔJ = 0, ±2. The reason for the values ±2 is that the polarizability returns to the same value twice during a rotation. The value ΔJ = 0 does not correspond to a molecular transition but rather to Rayleigh scattering
Rayleigh scattering ( ), named after the 19th-century British physicist Lord Rayleigh (John William Strutt), is the predominantly elastic scattering of light or other electromagnetic radiation by particles much smaller than the wavelength of the ...
in which the incident photon merely changes direction.
The selection rule for symmetric top molecules is
: Δ''K'' = 0
: If ''K'' = 0, then Δ''J'' = ±2
: If ''K'' ≠ 0, then Δ''J'' = 0, ±1, ±2
Transitions with Δ''J'' = +1 are said to belong to the ''R'' series, whereas transitions with belong to an ''S'' series. Since Raman transitions involve two photons, it is possible for the molecular angular momentum to change by two units.
Units
The units used for rotational constants depend on the type of measurement. With infrared spectra in thewavenumber
In the physical sciences, the wavenumber (also wave number or repetency) is the ''spatial frequency'' of a wave, measured in cycles per unit distance (ordinary wavenumber) or radians per unit distance (angular wavenumber). It is analogous to temp ...
scale (), the unit is usually the inverse centimeter, written as cm−1, which is literally the number of waves in one centimeter, or the reciprocal of the wavelength in centimeters (). On the other hand, for microwave spectra in the frequency scale (), the unit is usually the gigahertz
The hertz (symbol: Hz) is the unit of frequency in the International System of Units (SI), equivalent to one event (or cycle) per second. The hertz is an SI derived unit whose expression in terms of SI base units is s−1, meaning that one ...
. The relationship between these two units is derived from the expression
:
where ν is a frequency
Frequency is the number of occurrences of a repeating event per unit of time. It is also occasionally referred to as ''temporal frequency'' for clarity, and is distinct from '' angular frequency''. Frequency is measured in hertz (Hz) which is ...
, λ is a wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tr ...
and ''c'' is the velocity of light. It follows that
:
As 1 GHz = 109 Hz, the numerical conversion can be expressed as
:
Effect of vibration on rotation
The population of vibrationally excited states follows aBoltzmann distribution
In statistical mechanics and mathematics, a Boltzmann distribution (also called Gibbs distribution Translated by J.B. Sykes and M.J. Kearsley. See section 28) is a probability distribution or probability measure that gives the probability ...
, so low-frequency vibrational states are appreciably populated even at room temperatures. As the moment of inertia is higher when a vibration is excited, the rotational constants (''B'') decrease. Consequently, the rotation frequencies in each vibration state are different from each other. This can give rise to "satellite" lines in the rotational spectrum. An example is provided by cyanodiacetylene, H−C≡C−C≡C−C≡N.
Further, there is a fictitious force
A fictitious force is a force that appears to act on a mass whose motion is described using a non-inertial frame of reference, such as a linearly accelerating or rotating reference frame.
It is related to Newton's second law of motion, which t ...
, Coriolis coupling, between the vibrational motion of the nuclei in the rotating (non-inertial) frame. However, as long as the vibrational quantum number does not change (i.e., the molecule is in only one state of vibration), the effect of vibration on rotation is not important, because the time for vibration is much shorter than the time required for rotation. The Coriolis coupling is often negligible, too, if one is interested in low vibrational and rotational quantum numbers only.
Effect of rotation on vibrational spectra
Historically, the theory of rotational energy levels was developed to account for observations of vibration-rotation spectra of gases ininfrared spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
, which was used before microwave spectroscopy had become practical. To a first approximation, the rotation and vibration can be treated as separable, so the energy of rotation is added to the energy of vibration. For example, the rotational energy levels for linear molecules (in the rigid-rotor approximation) are
:
In this approximation, the vibration-rotation wavenumbers of transitions are
:
where and are rotational constants for the upper and lower vibrational state respectively, while and are the rotational quantum numbers of the upper and lower levels. In reality, this expression has to be modified for the effects of anharmonicity of the vibrations, for centrifugal distortion and for Coriolis coupling.
For the so-called ''R'' branch of the spectrum, so that there is simultaneous excitation of both vibration and rotation. For the ''P'' branch, so that a quantum of rotational energy is lost while a quantum of vibrational energy is gained. The purely vibrational transition, , gives rise to the ''Q'' branch of the spectrum. Because of the thermal population of the rotational states the ''P'' branch is slightly less intense than the ''R'' branch.
Rotational constants obtained from infrared measurements are in good accord with those obtained by microwave spectroscopy, while the latter usually offers greater precision.
Structure of rotational spectra
Spherical top
Spherical top molecules have no net dipole moment. A pure rotational spectrum cannot be observed by absorption or emission spectroscopy because there is no permanent dipole moment whose rotation can be accelerated by the electric field of an incident photon. Also the polarizability is isotropic, so that pure rotational transitions cannot be observed by Raman spectroscopy either. Nevertheless, rotational constants can be obtained by ro-vibrational spectroscopy. This occurs when a molecule is polar in the vibrationally excited state. For example, the moleculemethane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ear ...
is a spherical top but the asymmetric C-H stretching band shows rotational fine structure in the infrared spectrum, illustrated in rovibrational coupling. This spectrum is also interesting because it shows clear evidence of Coriolis coupling in the asymmetric structure of the band.
Linear molecules
 The
The rigid rotor
In rotordynamics, the rigid rotor is a mechanical model of rotating systems. An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top. To orient such an object in space requires three angles, known as Euler angles. A special ri ...
is a good starting point from which to construct a model of a rotating molecule. It is assumed that component atoms are point masses connected by rigid bonds. A linear molecule lies on a single axis and each atom moves on the surface of a sphere around the centre of mass. The two degrees of rotational freedom correspond to the spherical coordinates
In mathematics, a spherical coordinate system is a coordinate system for three-dimensional space where the position of a point is specified by three numbers: the ''radial distance'' of that point from a fixed origin, its ''polar angle'' mea ...
θ and φ which describe the direction of the molecular axis, and the quantum state is determined by two quantum numbers J and M. J defines the magnitude of the rotational angular momentum, and M its component about an axis fixed in space, such as an external electric or magnetic field. In the absence of external fields, the energy depends only on J. Under the rigid rotor
In rotordynamics, the rigid rotor is a mechanical model of rotating systems. An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top. To orient such an object in space requires three angles, known as Euler angles. A special ri ...
model, the rotational energy levels, ''F''(J), of the molecule can be expressed as,
:
where is the rotational constant of the molecule and is related to the moment of inertia of the molecule. In a linear molecule the moment of inertia about an axis perpendicular to the molecular axis is unique, that is, , so
:
For a diatomic molecule
:
where ''m''1 and ''m''2 are the masses of the atoms and ''d'' is the distance between them.
Selection rules
In physics and chemistry, a selection rule, or transition rule, formally constrains the possible transitions of a system from one quantum state to another. Selection rules have been derived for electromagnetic transitions in molecules, in atoms, i ...
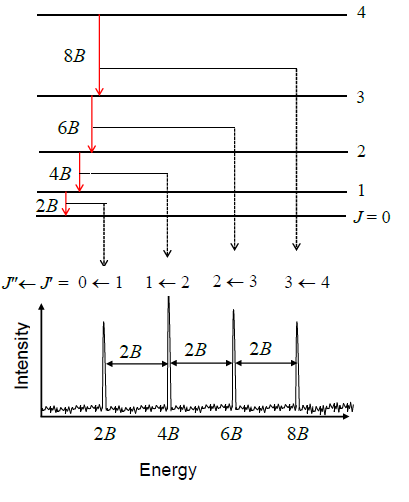
dictate that during emission or absorption the rotational quantum number has to change by unity; i.e., . Thus, the locations of the lines in a rotational spectrum will be given by
:
where denotes the lower level and denotes the upper level involved in the transition.
The diagram illustrates rotational transitions that obey the =1 selection rule. The dashed lines show how these transitions map onto features that can be observed experimentally. Adjacent transitions are separated by 2''B'' in the observed spectrum. Frequency or wavenumber units can also be used for the ''x'' axis of this plot.
Rotational line intensities
The probability of a transition taking place is the most important factor influencing the intensity of an observed rotational line. This probability is proportional to the population of the initial state involved in the transition. The population of a rotational state depends on two factors. The number of molecules in an excited state with quantum number ''J'', relative to the number of molecules in the ground state, ''NJ''/''N''0 is given by theBoltzmann distribution
In statistical mechanics and mathematics, a Boltzmann distribution (also called Gibbs distribution Translated by J.B. Sykes and M.J. Kearsley. See section 28) is a probability distribution or probability measure that gives the probability ...
as
:,
where ''k'' is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas consta ...
and ''T'' the absolute temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.
Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic ...
. This factor decreases as ''J'' increases. The second factor is the degeneracy of the rotational state, which is equal to . This factor increases as ''J'' increases. Combining the two factors
:
The maximum relative intensity occurs atThis value of J corresponds to the maximum of the population considered as a continuous function of J. However, since only integer values of J are allowed, the maximum line intensity is observed for a neighboring integer J.
:
The diagram at the right shows an intensity pattern roughly corresponding to the spectrum above it.
Centrifugal distortion
When a molecule rotates, thecentrifugal force
In Newtonian mechanics, the centrifugal force is an inertial force (also called a "fictitious" or "pseudo" force) that appears to act on all objects when viewed in a rotating frame of reference. It is directed away from an axis which is parallel ...
pulls the atoms apart. As a result, the moment of inertia of the molecule increases, thus decreasing the value of , when it is calculated using the expression for the rigid rotor. To account for this a centrifugal distortion correction term is added to the rotational energy levels of the diatomic molecule.
:
where is the centrifugal distortion constant.
Therefore, the line positions for the rotational mode change to
:
In consequence, the spacing between lines is not constant, as in the rigid rotor approximation, but decreases with increasing rotational quantum number.
An assumption underlying these expressions is that the molecular vibration follows simple harmonic motion
In mechanics and physics, simple harmonic motion (sometimes abbreviated ) is a special type of periodic motion of a body resulting from a dynamic equilibrium between an inertial force, proportional to the acceleration of the body away from the ...
. In the harmonic approximation the centrifugal constant can be derived as
:
where ''k'' is the vibrational force constant. The relationship between and
:
where is the harmonic vibration frequency, follows. If anharmonicity is to be taken into account, terms in higher powers of J should be added to the expressions for the energy levels and line positions. A striking example concerns the rotational spectrum of hydrogen fluoride which was fitted to terms up to '' (J+1)sup>5''.
Oxygen
The electric dipole moment of the dioxygen molecule, is zero, but the molecule isparamagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
with two unpaired electrons so that there are magnetic-dipole allowed transitions which can be observed by microwave spectroscopy. The unit electron spin has three spatial orientations with respect to the given molecular rotational angular momentum vector, K, so that each rotational level is split into three states, J = K + 1, K, and K - 1, each J state of this so-called p-type triplet arising from a different orientation of the spin with respect to the rotational motion of the molecule. The energy difference between successive J terms in any of these triplets is about 2 cm−1 (60 GHz), with the single exception of J = 1←0 difference which is about 4 cm−1. Selection rules for magnetic dipole transitions allow transitions between successive members of the triplet (ΔJ = ±1) so that for each value of the rotational angular momentum quantum number K there are two allowed transitions. The 16O nucleus has zero nuclear spin angular momentum, so that symmetry considerations demand that K have only odd values.
Symmetric top
For symmetric rotors a quantum number ''J'' is associated with the total angular momentum of the molecule. For a given value of J, there is a 2''J''+1- fold degeneracy with the quantum number, ''M'' taking the values +''J'' ...0 ... -''J''. The third quantum number, ''K'' is associated with rotation about the principal rotation axis of the molecule. In the absence of an external electrical field, the rotational energy of a symmetric top is a function of only J and K and, in the rigid rotor approximation, the energy of each rotational state is given by : where and for a ''prolate'' symmetric top molecule or for an ''oblate'' molecule. This gives the transition wavenumbers as : which is the same as in the case of a linear molecule. With a first order correction for centrifugal distortion the transition wavenumbers become : The term in ''DJK'' has the effect of removing degeneracy present in the rigid rotor approximation, with different ''K'' values.Asymmetric top
matrix diagonalization
In linear algebra, a square matrix A is called diagonalizable or non-defective if it is similar to a diagonal matrix, i.e., if there exists an invertible matrix P and a diagonal matrix D such that or equivalently (Such D are not unique.) F ...
for each ''J'' value. Formulae are available for molecules whose shape approximates to that of a symmetric top.
The water molecule is an important example of an asymmetric top. It has an intense pure rotation spectrum in the far infrared region, below about 200 cm−1. For this reason far infrared spectrometers have to be freed of atmospheric water vapour either by purging with a dry gas or by evacuation. The spectrum has been analyzed in detail.
Quadrupole splitting
When a nucleus has aspin quantum number
In atomic physics, the spin quantum number is a quantum number (designated ) which describes the intrinsic angular momentum (or spin angular momentum, or simply spin) of an electron or other particle. The phrase was originally used to describe ...
, ''I'', greater than 1/2 it has a quadrupole
A quadrupole or quadrapole is one of a sequence of configurations of things like electric charge or current, or gravitational mass that can exist in ideal form, but it is usually just part of a multipole expansion of a more complex structure ref ...
moment. In that case, coupling of nuclear spin angular momentum with rotational angular momentum causes splitting of the rotational energy levels. If the quantum number ''J'' of a rotational level is greater than ''I'', levels are produced; but if ''J'' is less than ''I'', levels result. The effect is one type of hyperfine splitting
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to electromagnetic multipole interaction between the nu ...
. For example, with 14N () in HCN, all levels with J > 0 are split into 3. The energies of the sub-levels are proportional to the nuclear quadrupole moment Nuclear quadrupole resonance spectroscopy or NQR is a chemical analysis technique related to nuclear magnetic resonance ( NMR). Unlike NMR, NQR transitions of nuclei can be detected in the absence of a magnetic field, and for this reason NQR spectr ...
and a function of ''F'' and ''J''. where , . Thus, observation of nuclear quadrupole splitting permits the magnitude of the nuclear quadrupole moment to be determined.
This is an alternative method to the use of nuclear quadrupole resonance Nuclear quadrupole resonance spectroscopy or NQR is a chemical analysis technique related to nuclear magnetic resonance ( NMR). Unlike NMR, NQR transitions of nuclei can be detected in the absence of a magnetic field, and for this reason NQR spectro ...
spectroscopy. The selection rule for rotational transitions becomes
:
Stark and Zeeman effects
In the presence of a static external electric field the degeneracy of each rotational state is partly removed, an instance of aStark effect
The Stark effect is the shifting and splitting of spectral lines of atoms and molecules due to the presence of an external electric field. It is the electric-field analogue of the Zeeman effect, where a spectral line is split into several compo ...
. For example, in linear molecules each energy level is split into components. The extent of splitting depends on the square of the electric field strength and the square of the dipole moment of the molecule. In principle this provides a means to determine the value of the molecular dipole moment with high precision. Examples include carbonyl sulfide
Carbonyl sulfide is the chemical compound with the linear formula OCS. It is a colorless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl group double bonded to a sulfur atom. Carbonyl sulfide can be consid ...
, OCS, with . However, because the splitting depends on μ2, the orientation of the dipole must be deduced from quantum mechanical considerations.
A similar removal of degeneracy will occur when a paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
molecule is placed in a magnetic field, an instance of the Zeeman effect
The Zeeman effect (; ) is the effect of splitting of a spectral line into several components in the presence of a static magnetic field. It is named after the Dutch physicist Pieter Zeeman, who discovered it in 1896 and received a Nobel priz ...
. Most species which can be observed in the gaseous state are diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
. Exceptions are odd-electron molecules such as nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its ...
, NO, nitrogen dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the productio ...
, , some chlorine oxides and the hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
. The Zeeman effect has been observed with dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are: ...
,
Rotational Raman spectroscopy
Molecular rotational transitions can also be observed byRaman spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman s ...
. Rotational transitions are Raman-allowed for any molecule with an anisotropic
Anisotropy () is the property of a material which allows it to change or assume different properties in different directions, as opposed to isotropy. It can be defined as a difference, when measured along different axes, in a material's phys ...
polarizability
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of all matter, considering that matter is made up of elementar ...
which includes all molecules except for spherical tops. This means that rotational transitions of molecules with no permanent dipole moment, which cannot be observed in absorption or emission, can be observed, by scattering, in Raman spectroscopy. Very high resolution Raman spectra can be obtained by adapting a Fourier Transform Infrared Spectrometer. An example is the spectrum of . It shows the effect of nuclear spin, resulting in intensities variation of 3:1 in adjacent lines. A bond length of 109.9985 ± 0.0010 pm was deduced from the data.
Instruments and methods
The great majority of contemporary spectrometers use a mixture of commercially available and bespoke components which users integrate according to their particular needs. Instruments can be broadly categorised according to their general operating principles. Although rotational transitions can be found across a very broad region of theelectromagnetic spectrum
The electromagnetic spectrum is the range of frequencies (the spectrum) of electromagnetic radiation and their respective wavelengths and photon energies.
The electromagnetic spectrum covers electromagnetic waves with frequencies ranging from ...
, fundamental physical constraints exist on the operational bandwidth of instrument components. It is often impractical and costly to switch to measurements within an entirely different frequency region. The instruments and operating principals described below are generally appropriate to microwave spectroscopy experiments conducted at frequencies between 6 and 24 GHz.
Absorption cells and Stark modulation
A microwave spectrometer can be most simply constructed using a source of microwave radiation, an absorption cell into which sample gas can be introduced and a detector such as asuperheterodyne receiver
A superheterodyne receiver, often shortened to superhet, is a type of radio receiver that uses frequency mixing to convert a received signal to a fixed intermediate frequency (IF) which can be more conveniently processed than the original carr ...
. A spectrum can be obtained by sweeping the frequency of the source while detecting the intensity of transmitted radiation. A simple section of waveguide
A waveguide is a structure that guides waves, such as electromagnetic waves or sound, with minimal loss of energy by restricting the transmission of energy to one direction. Without the physical constraint of a waveguide, wave intensities de ...
can serve as an absorption cell. An important variation of the technique in which an alternating current is applied across electrodes within the absorption cell results in a modulation of the frequencies of rotational transitions. This is referred to as Stark modulation and allows the use of phase-sensitive detection methods offering improved sensitivity. Absorption spectroscopy allows the study of samples that are thermodynamically stable at room temperature.
The first study of the microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequency, frequencies between 300 MHz and 300 GHz respectively. Different sources define different fre ...
spectrum of a molecule () was performed by Cleeton & Williams in 1934. Subsequent experiments exploited powerful sources of microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequency, frequencies between 300 MHz and 300 GHz respectively. Different sources define different fre ...
s such as the klystron
A klystron is a specialized linear-beam vacuum tube, invented in 1937 by American electrical engineers Russell and Sigurd Varian,Pond, Norman H. "The Tube Guys". Russ Cochran, 2008 p.31-40 which is used as an amplifier for high radio frequen ...
, many of which were developed for radar
Radar is a detection system that uses radio waves to determine the distance ('' ranging''), angle, and radial velocity of objects relative to the site. It can be used to detect aircraft, ships, spacecraft, guided missiles, motor vehicles, w ...
during the Second World War
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the World War II by country, vast majority of the world's countries—including all of the great power ...
. The number of experiments in microwave spectroscopy surged immediately after the war. By 1948, Walter Gordy
Walter Gordy, (April 20, 1909 – October 6, 1985) was an American physicist best known for his experimental work in microwave spectroscopy. His laboratory at Duke University became a center for research in this field, and he authored one of t ...
was able to prepare a review of the results contained in approximately 100 research papers. Commercial versions of microwave absorption spectrometer were developed by Hewlett Packard
The Hewlett-Packard Company, commonly shortened to Hewlett-Packard ( ) or HP, was an American multinational information technology company headquartered in Palo Alto, California. HP developed and provided a wide variety of hardware components ...
in the 1970s and were once widely used for fundamental research. Most research laboratories now exploit either Balle- Flygare or chirped-pulse Fourier transform microwave (FTMW) spectrometers.
Fourier transform microwave (FTMW) spectroscopy
The theoretical framework underpinning FTMW spectroscopy is analogous to that used to describe FT-NMR spectroscopy. The behaviour of the evolving system is described by opticalBloch equations
In physics and chemistry, specifically in nuclear magnetic resonance (NMR), magnetic resonance imaging (MRI), and electron spin resonance (ESR), the Bloch equations are a set of macroscopic equations that are used to calculate the nuclear magneti ...
. First, a short (typically 0-3 microsecond duration) microwave pulse is introduced on resonance with a rotational transition. Those molecules that absorb the energy from this pulse are induced to rotate coherently in phase with the incident radiation. De-activation of the polarisation pulse is followed by microwave emission that accompanies decoherence of the molecular ensemble. This free induction decay
In Fourier transform nuclear magnetic resonance spectroscopy, free induction decay (FID) is the observable NMR signal generated by non-equilibrium nuclear spin magnetization precessing about the magnetic field (conventionally along z). This non-e ...
occurs on a timescale of 1-100 microseconds depending on instrument settings. Following pioneering work by Dicke and co-workers in the 1950s, the first FTMW spectrometer was constructed by Ekkers and Flygare in 1975.
Balle-Flygare FTMW spectrometer
Balle, Campbell, Keenan and Flygare demonstrated that the FTMW technique can be applied within a "free space cell" comprising an evacuated chamber containing a Fabry-Perot cavity. This technique allows a sample to be probed only milliseconds after it undergoes rapid cooling to only a fewkelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and ph ...
s in the throat of an expanding gas jet. This was a revolutionary development because (i) cooling molecules to low temperatures concentrates the available population in the lowest rotational energy levels. Coupled with benefits conferred by the use of a Fabry-Perot cavity, this brought a great enhancement in the sensitivity and resolution of spectrometers along with a reduction in the complexity of observed spectra; (ii) it became possible to isolate and study molecules that are very weakly bound because there is insufficient energy available for them to undergo fragmentation or chemical reaction at such low temperatures. William Klemperer
William A. Klemperer (October 6, 1927 – November 5, 2017) was an American chemist who was one of the most influential chemical physicists and molecular spectroscopists in the second half of the 20th century. Klemperer is most widely known fo ...
was a pioneer in using this instrument for the exploration of weakly bound interactions. While the Fabry-Perot cavity of a Balle-Flygare FTMW spectrometer can typically be tuned into resonance at any frequency between 6 and 18 GHz, the bandwidth of individual measurements is restricted to about 1 MHz. An animation illustrates the operation of this instrument which is currently the most widely used tool for microwave spectroscopy.
Chirped-Pulse FTMW spectrometer
Noting that digitisers and related electronics technology had significantly progressed since the inception of FTMW spectroscopy, B.H. Pate at the University of Virginia designed a spectrometer which retains many advantages of the Balle-Flygare FT-MW spectrometer while innovating in (i) the use of a high speed (>4 GS/s) arbitrary waveform generator to generate a "chirped" microwave polarisation pulse that sweeps up to 12 GHz in frequency in less than a microsecond and (ii) the use of a high speed (>40 GS/s) oscilloscope to digitise and Fourier transform the molecular free induction decay. The result is an instrument that allows the study of weakly bound molecules but which is able to exploit a measurement bandwidth (12 GHz) that is greatly enhanced compared with the Balle-Flygare FTMW spectrometer. Modified versions of the original CP-FTMW spectrometer have been constructed by a number of groups in the United States, Canada and Europe. The instrument offers a broadband capability that is highly complementary to the high sensitivity and resolution offered by the Balle-Flygare design.Notes
References
Bibliography
* * * * * * * * *External links
infrared gas spectra simulator
{{Branches of spectroscopy Spectroscopy Rotation Rigid bodies mechanics