Sonogashira Coupling on:
[Wikipedia]
[Google]
[Amazon]
The Sonogashira reaction is a
 The
The
 This difference in reactivity can be exploited to selectively couple an aryl iodide but not an aryl bromide, by performing the reaction at room temperature. An example is the symmetrical Sonogashira coupling of two equivalents of 1-bromo-4-iodobenzene with
This difference in reactivity can be exploited to selectively couple an aryl iodide but not an aryl bromide, by performing the reaction at room temperature. An example is the symmetrical Sonogashira coupling of two equivalents of 1-bromo-4-iodobenzene with  Aryl triflates can also be employed instead of aryl halides.
Aryl triflates can also be employed instead of aryl halides.
 The dendrimeric catalysts could usually be recovered by simple precipitation and filtration and reused up to five times, with diminished activity produced by dendrimer decomposition and not by palladium leaching being observed. These dendrimeric catalysts showed a negative dendritic effect; that is, the catalyst efficiency decreases as the dendrimer generation increases. A recyclable polymeric phosphine ligand is obtained from ring-opening metathesis polymerization of a norbornene derivative, and has been used in the copper co-catalyzed Sonogashira reaction of methyl ''p''-iodobenzoate and phenylacetylene using as a palladium source. Despite recovery by filtration, polymer catalytic activity decreased by approximately 4-8% in each recycle experiment.
The dendrimeric catalysts could usually be recovered by simple precipitation and filtration and reused up to five times, with diminished activity produced by dendrimer decomposition and not by palladium leaching being observed. These dendrimeric catalysts showed a negative dendritic effect; that is, the catalyst efficiency decreases as the dendrimer generation increases. A recyclable polymeric phosphine ligand is obtained from ring-opening metathesis polymerization of a norbornene derivative, and has been used in the copper co-catalyzed Sonogashira reaction of methyl ''p''-iodobenzoate and phenylacetylene using as a palladium source. Despite recovery by filtration, polymer catalytic activity decreased by approximately 4-8% in each recycle experiment.

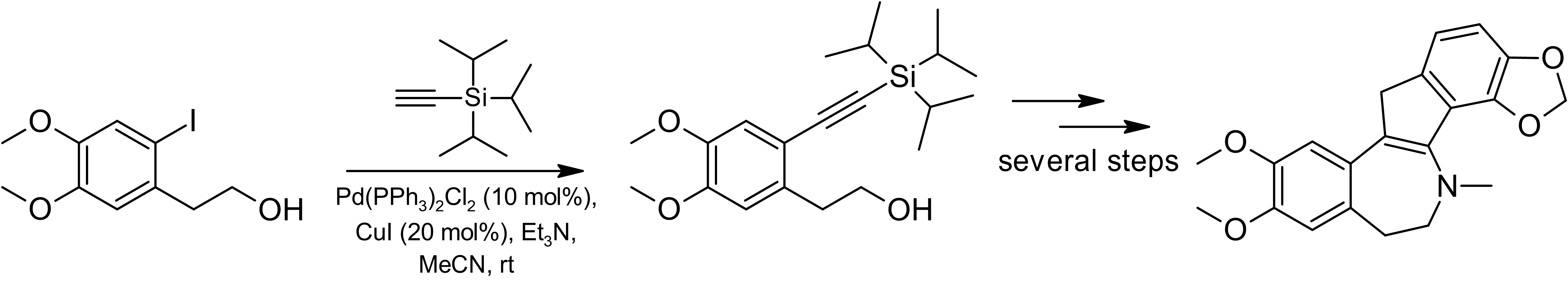 There are other recent examples of the use of aryl iodides for the preparation of intermediates under typical Sonogashira conditions, which, after cyclization, yield natural products such as benzylisoquinoline or indole alkaloids An example is the synthesis of the benzylisoquinoline alkaloids (+)-(''S'')- laudanosine and (–)-(''S'')-xylopinine. The synthesis of these natural products involved the use of Sonogashira cross-coupling to build the carbon backbone of each molecule.
There are other recent examples of the use of aryl iodides for the preparation of intermediates under typical Sonogashira conditions, which, after cyclization, yield natural products such as benzylisoquinoline or indole alkaloids An example is the synthesis of the benzylisoquinoline alkaloids (+)-(''S'')- laudanosine and (–)-(''S'')-xylopinine. The synthesis of these natural products involved the use of Sonogashira cross-coupling to build the carbon backbone of each molecule.

 The Sonogashira cross coupling reaction can be used in the synthesis of imidazopyridine derivatives.
The Sonogashira cross coupling reaction can be used in the synthesis of imidazopyridine derivatives.

cross-coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two different fragments are joined. Cross-couplings are a subset of the more general coupling reactions. Often cross-coupling reactions require metal catalysts. One important re ...
used in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
to form carbon–carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed between on ...
s. It employs a palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
as well as copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
co-catalyst to form a carbon–carbon bond between a terminal alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
and an aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
or vinyl halide.
:* R1: aryl or vinyl
:* R2: arbitrary
:* X: I, Br, Cl or OTf
The Sonogashira cross-coupling reaction has been employed in a wide variety of areas, due to its usefulness in the formation of carbon–carbon bonds. The reaction can be carried out under mild conditions, such as at room temperature, in aqueous media, and with a mild base, which has allowed for the use of the Sonogashira cross-coupling reaction in the synthesis of complex molecules. Its applications include pharmaceuticals, natural products, organic materials, and nanomaterials
Nanomaterials describe, in principle, chemical substances or materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale).
Nanomaterials research takes a materials science ...
. Specific examples include its use in the synthesis of tazarotene
Tazarotene, sold under the brand name Tazorac, among others, is a third-generation prescription topical retinoid. It is primarily used for the treatment of plaque psoriasis and acne. Tazarotene is also used as a therapeutic for photoaged and p ...
, which is a treatment for psoriasis
Psoriasis is a long-lasting, noncontagious autoimmune disease characterized by patches of abnormal skin. These areas are red, pink, or purple, dry, itchy, and scaly. Psoriasis varies in severity from small localized patches to complete b ...
and acne
Acne ( ), also known as ''acne vulgaris'', is a long-term Cutaneous condition, skin condition that occurs when Keratinocyte, dead skin cells and Sebum, oil from the skin clog hair follicles. Typical features of the condition include comedo, ...
, and in the preparation of SIB-1508Y, also known as Altinicline, a nicotinic receptor agonist
An agonist is a chemical that activates a Receptor (biochemistry), receptor to produce a biological response. Receptors are Cell (biology), cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an R ...
.
History
The alkynylation reaction of aryl halides using aromatic acetylenes was reported in 1975 in three independent contributions by Cassar, Dieck and Heck as well as Sonogashira, Tohda and Hagihara. All of the reactions employ palladium catalysts to afford the same reaction products. However, the protocols of Cassar and Heck are performed solely by the use of palladium and require harsh reaction conditions (i.e. high reaction temperatures). The use of copper-cocatalyst in addition to palladium complexes in Sonogashira's procedure enabled the reactions to be carried under mild reaction conditions in excellent yields. A rapid development of the Pd/Cu systems followed and enabled myriad synthetic applications, while Cassar-Heck conditions were left, maybe unjustly, all but forgotten. The reaction's remarkable utility can be evidenced by the amount of research still being done on understanding and optimizing its synthetic capabilities as well as employing the procedures to prepare various compounds of synthetic, medicinal or material/industrial importance. Among the cross-coupling reactions it follows in the number of publications right after Suzuki and Heck reaction and a search for the term "Sonogashira" inSciFinder
Chemical Abstracts Service (CAS) is a division of the American Chemical Society. It is a source of chemical information and is located in Columbus, Ohio, United States.
Print periodicals
''Chemical Abstracts'' is a periodical index that provid ...
provides over 1500 references for journal publications between 2007 and 2010.
The Sonogashira reaction has become so well known that often all reactions that use modern organometallic catalyst to couple alkyne motifs are termed some variant of "Sonogashira reaction", despite the fact that these reactions are not carried out under true Sonogashira reaction conditions.
Mechanism
 The
The reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
is not clearly understood, but the textbook mechanism revolves around a palladium cycle which is in agreement with the "classical" cross-coupling mechanism, and a copper cycle, which is less well known.
The palladium cycle
* Palladium precatalyst species are activated under reaction conditions to form a reactive Pd0 compound, A. The exact identity of the catalytic species depends strongly upon reaction conditions. With simple phosphines, such as PPh3 (n=2), and in case of bulky phosphines (i.e., ) it was demonstrated that monoligated species (n=1) are formed. Furthermore, some results point to the formation of anionic palladium species, 2Pd0Clsup>− , which could be the real catalysts in the presence of anions and halides. * The active Pd0 catalyst is involved in the oxidative addition step with thearyl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
or vinyl halide substrate to produce PdII species B. Similar to the above discussion, its structure depends on the employed ligands. This step is believed to be the rate-limiting step of the reaction.
* Complex B reacts with copper acetylide, complex F, in a transmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
step, yielding complex C and regenerating the copper catalyst.
* The structure of complex C depends on the properties of the ligands. For the facile reductive elimination to occur, the substrate motifs need to be in close vicinity, i.e. cis-orientation, so there can be trans-cis isomerisation involved. In reductive elimination the product tolane is expelled from the complex and the active Pd catalytic species is regenerated.
The copper cycle
* The copper cycle is not entirely well described. It is suggested that the presence of a base results in the formation of a π-alkyne complex E. This increases the acidity of the terminal proton and leads to the formation of copper acetylide, complex F, upon deprotonation. * Acetylide F is then involved in the transmetallation reaction with palladium intermediate B.The mechanism of a copper-free Sonogashira variant
Although beneficial for the effectiveness of the reaction, the use of copper salts in "classical" Sonogashira reaction is accompanied with several drawbacks, such as the application of environmentally unfriendly reagents, the formation of undesirable alkyne homocoupling ( Glaser side products), and the necessity of strict oxygen exclusion in the reaction mixture. Thus, with the aim of excluding copper from the reaction, a lot of effort was undertaken in the developments of Cu-free Sonogashira reaction. Along the development of new reaction conditions, many experimental and computational studies focused on elucidation of reaction mechanism. Until recently, the exact mechanism by which the Cu-free reaction occurs was under debate, with critical mechanistic questions unanswered. It was shown in 2018 by Košmrlj et al. that the reaction proceeds along the two interconnected Pd0/PdII catalytic cycles. * Similar to the original mechanism, the Pd0 cycle begins with the oxidative addition of the aryl halide or triflate to the Pd0 catalyst, forming complex B and activating aryl halide substrate for the reaction. * Acetylene is activated in the second, PdII mediated cycle. Phenylacetylene was proven to form Pd monoacetylide complex D as well as Pd bisacetylide complex F under mild reaction conditions. * Both activated species, namely complexes B and F, are involved in thetransmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
step, forming complex C and regenerating D.
* The resulting products of reductive elimination, disubstituted alkyne product as well as regenerated Pd0 catalytic species, complete the Pd0 catalytic cycle.
It was demonstrated that amines are competitive to the phosphines and can also participate as ligands L in the described reaction species. Depending on the rate of the competition between amine and phosphines, a dynamic and complex interplay is expected when using different coordinative bases.
Reaction conditions
The Sonogashira reaction is typically run under mild conditions. The cross-coupling is carried out at room temperature with a base, typically an amine, such asdiethylamine
Diethylamine is an organic compound with the formula . It is classified as a secondary amine. It is a flammable, volatile weakly alkaline liquid that is miscible with most solvents. It is a colorless liquid, but commercial samples often appear br ...
, that also acts as the solvent. The reaction medium must be basic to neutralize the hydrogen halide produced as the byproduct of this coupling reaction, so alkylamine compounds such as triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
and diethylamine
Diethylamine is an organic compound with the formula . It is classified as a secondary amine. It is a flammable, volatile weakly alkaline liquid that is miscible with most solvents. It is a colorless liquid, but commercial samples often appear br ...
are sometimes used as solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
s, but also DMF or ether can be used as solvent. Other bases such as potassium carbonate or cesium carbonate are occasionally used. In addition, deaerated conditions are formally needed for Sonogashira coupling reactions because the palladium(0) complexes are unstable in the air, and oxygen promotes the formation of homocoupled acetylenes. Recently, development of air-stable organopalladium catalysts enable this reaction to be conducted in the ambient atmosphere. In addition, R.M Al-Zoubi and co-workers successfully developed a method with high regioselectivity for 1,2,3-trihaloarene derivatives in good to high yields under ambient conditions.
Catalysts
Typically, two catalysts are needed for this reaction: a zerovalent palladium complex and a copper(I) halide salt. Common examples of palladium catalysts include those containing phosphine ligands such as . Another commonly used palladium source is bidentate phosphine ligands, such as , , and (1,1'-Bis(diphenylphosphino)ferrocene)palladium(II) dichloride"> have also been used. The drawback to such catalysts is the need for high loadings of palladium (up to 5 mol %), along with a larger amount of a copper co-catalyst. PdII complexes are in fact pre-catalysts since they must be reduced to Pd0 before catalysis can begin. PdII complexes generally exhibit greater stability than Pd0 complexes and can be stored under normal laboratory conditions for months. PdII catalysts are reduced to Pd0 in the reaction mixture by anamine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
, a phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
ligand, or another reactant in the mixture allowing the reaction to proceed. For instance, oxidation of triphenylphosphine to triphenylphosphine oxide can lead to the formation of Pd0 ''in situ
is a Latin phrase meaning 'in place' or 'on site', derived from ' ('in') and ' ( ablative of ''situs'', ). The term typically refers to the examination or occurrence of a process within its original context, without relocation. The term is use ...
'' when is used.
Copper(I) salts, such as CuI, react with the terminal alkyne and produce a copper(I) acetylide, which acts as an activated species for the coupling reactions. Cu(I) is a co-catalyst in the reaction, and is used to increase the rate of the reaction.
Aryl halides and pseudohalides
The choice of aryl halide or pseudohalide substrate (sp2-carbon) is one of the factors that mainly influence the reactivity of the Sonogashira catalytic system. The reactivity of halides is higher towards iodine, and vinyl halides are more reactive than analogous aryl halides. The coupling of aryl iodides proceeds at room temperature, while aryl bromides require heating.trimethylsilylacetylene
Trimethylsilylacetylene is the organosilicon compound with the formula . A colorless liquid, "tms acetylene", as it is also called, is used as a source of anion in organic synthesis.
Use
Trimethylsilylacetylene is used in Sonogashira couplin ...
(with the trimethylsilyl group
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group i ...
removed ''in-situ'') to form bis(4-bromophenyl)acetylene.
 Aryl triflates can also be employed instead of aryl halides.
Aryl triflates can also be employed instead of aryl halides.
Arenediazonium precursors
Arenediazonium salts have been reported as an alternative to aryl halides for the Sonogashira coupling reaction. Gold(I) chloride has been used as co-catalyst combined with palladium(II) chloride in the coupling of arenediazonium salts with terminal alkynes, a process carried out in the presence of bis-2,6-diisopropylphenyl dihydroimidazolium chloride (IPr NHC) (5 mol%) to ''in situ'' generate a NHC–palladium complex, and 2,6-di-tert-butyl-4-methylpyridine (DBMP) as base in acetonitrile as solvent at room temperature. This coupling can be carried out starting from anilines by formation of the diazonium salt followed by ''in situ'' Sonogashira coupling, where anilines are transformed into diazonium salt and furtherly converted into alkyne by coupling with phenylacetylene.Alkynes
Various aromatic alkynes can be employed to yield desired disubstituted products with satisfactory yields. Aliphatic alkynes are generally less reactive.Bases
Due to the crucial role of base, specific amines must be added in excess or as solvent for the reaction to proceed. It has been discovered that secondary amines such as piperidine, morpholine, or diisopropylamine in particular can react efficiently and reversibly with ''trans''– complexes by substituting one ligand. The equilibrium constant of this reaction is dependent on R, X, a factor for basicity, and the amine's steric hindrance. The result is competition between the amine and the alkyne group for this ligand exchange, which is why the amine is generally added in excess to promote preferential substitution.Protecting groups
Trimethylsilylacetylene
Trimethylsilylacetylene is the organosilicon compound with the formula . A colorless liquid, "tms acetylene", as it is also called, is used as a source of anion in organic synthesis.
Use
Trimethylsilylacetylene is used in Sonogashira couplin ...
is a commonly used reagent in Sonogashira couplings. Being a liquid it is a more convenient reagent than the gaseous acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
, and the trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group i ...
group prevents addition onto the other end of the acetylene group. The trimethylsilyl group can then be removed using TBAF, yielding a monosubstituted acetylene. It may also be removed using DBU in situ, allowing the monosubstituted acetylene to react further with another aryl halide to form diphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a Transition metal alkyne complex, ligand in ...
and derivatives.
Reaction variations
Copper-free Sonogashira coupling
While a copper co-catalyst is added to the reaction to increase reactivity, the presence of copper can result in the formation of alkyne dimers. This leads to what is known as the Glaser coupling reaction, which is an undesired formation of homocoupling products of acetylene derivatives uponoxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
. As a result, when running a Sonogashira reaction with a copper co-catalyst, it is necessary to run the reaction in an inert atmosphere to avoid the unwanted dimerization. Copper-free variations to the Sonogashira reaction have been developed to avoid the formation of the homocoupling products. There are other cases when the use of copper should be avoided, such as coupling reactions involving substrates which potential copper ligands, for instance free-base porphyrins.
Inverse Sonogashira coupling
In an inverse Sonogashira coupling the reactants are an aryl or vinyl compound and an alkynyl halide.Catalyst variations
Silver co-catalysis
In some cases stoichiometric amounts of silver oxide can be used in place of CuI for copper-free Sonogashira couplings.Nickel catalysts
Recently, a nickel-catalyzed Sonogashira coupling has been developed which allows for the coupling of non-activated alkyl halides to acetylene without the use of palladium, although a copper co-catalyst is still needed. It has also been reported that gold can be used as a heterogeneous catalyst, which was demonstrated in the coupling of phenylacetylene and iodobenzene with an Au/CeO2 catalyst. In this case, catalysis occurs heterogeneously on the Au nanoparticles, with Au(0) as the active site. Selectivity to the desirable cross coupling product was also found to be enhanced by supports such as CeO2 and La2O3. Additionally, iron-catalyzed Sonogashira couplings have been investigated as relatively cheap and non-toxic alternatives to palladium. Here, FeCl3 is proposed to act as the transition-metal catalyst and Cs2CO3 as the base, thus theoretically proceeding through a palladium-free and copper-free mechanism. While the copper-free mechanism has been shown to be viable, attempts to incorporate the various transition metals mentioned above as less expensive alternatives to palladium catalysts have shown a poor track record of success due to contamination of the reagents with trace amounts of palladium, suggesting that these theorized pathways are extremely unlikely, if not impossible, to achieve. Studies have shown that organic and inorganic starting materials can also contain enough ( ppb level) palladium for the coupling.Gold and palladium co-catalysis
A highly efficient gold and palladium combined methodology for the Sonogashira coupling of a wide array of electronically and structurally diverse aryl and heteroaryl halides has been reported. The orthogonal reactivity of the two metals shows high selectivity and extreme functional group tolerance in Sonogashira coupling. A brief mechanistic study reveals that the gold-acetylide intermediate enters into palladium catalytic cycle at the transmetalation step.Dendrimeric palladium complexes
The issues dealing with recovery of the often expensive catalyst after product formation poses a serious drawback for large-scale applications of homogeneous catalysis. Structures known as metallodendrimers combine the advantages of homogeneous and heterogeneous catalysts, as they are soluble and well defined on the molecular level, and yet they can be recovered by precipitation, ultrafiltration, or ultracentrifugation. Some recent examples can be found about the use of dendritic palladium complex catalysts for the copper-free Sonogashira reaction. Thus, several generations of bidentate phosphine palladium(II) polyamino dendritic catalysts have been used solubilized in triethylamine for the coupling of aryl iodides and bromides at 25-120 °C, and of aryl chlorides, but in very low yields. The dendrimeric catalysts could usually be recovered by simple precipitation and filtration and reused up to five times, with diminished activity produced by dendrimer decomposition and not by palladium leaching being observed. These dendrimeric catalysts showed a negative dendritic effect; that is, the catalyst efficiency decreases as the dendrimer generation increases. A recyclable polymeric phosphine ligand is obtained from ring-opening metathesis polymerization of a norbornene derivative, and has been used in the copper co-catalyzed Sonogashira reaction of methyl ''p''-iodobenzoate and phenylacetylene using as a palladium source. Despite recovery by filtration, polymer catalytic activity decreased by approximately 4-8% in each recycle experiment.
The dendrimeric catalysts could usually be recovered by simple precipitation and filtration and reused up to five times, with diminished activity produced by dendrimer decomposition and not by palladium leaching being observed. These dendrimeric catalysts showed a negative dendritic effect; that is, the catalyst efficiency decreases as the dendrimer generation increases. A recyclable polymeric phosphine ligand is obtained from ring-opening metathesis polymerization of a norbornene derivative, and has been used in the copper co-catalyzed Sonogashira reaction of methyl ''p''-iodobenzoate and phenylacetylene using as a palladium source. Despite recovery by filtration, polymer catalytic activity decreased by approximately 4-8% in each recycle experiment.
Nitrogen ligands
Pyridines and pyrimidines have shown good complexation properties for palladium and have been employed in the formation of catalysts suitable for Sonogashira couplings. The dipyrimidyl-palladium complex shown below has been employed in the copper-free coupling of iodo-, bromo-, and chlorobenzene with phenylacetylene using N-butylamine as base in THF solvent at 65 °C. Furthermore, all structural features of this complex have been characterized by extensive X-ray analysis, verifying the observed reactivity. More recently, the dipyridylpalladium complex has been obtained and has been used in the copper-free Sonogashira coupling reaction of aryl iodides and bromides in ''N''-methylpyrrolidinone (NMP) using tetra-n-butylammonium acetate (TBAA) as base at room temperature. This complex has also been used for the coupling of aryl iodides and bromides in refluxing water as solvent and in the presence of air, using pyrrolidine as base and TBAB as additive, although its efficiency was higher in ''N''-methylpyrrolidinone (NMP) as solvent.''N''-heterocyclic carbene (NHC) palladium complexes
''N''-heterocyclic carbenes (NHCs) have become one of the most important ligands in transition-metal catalysis. The success of normal NHCs is greatly attributed to their superior σ-donating capabilities as compared to phosphines, which is even greater in abnormal NHC counterparts. Employed as ligands in palladium complexes, NHCs contributed greatly to the stabilization and activation of precatalysts and have therefore found application in many areas of organometallic homogeneous catalysis, including Sonogashira couplings. Interesting examples of abnormal NHCs are based on the mesoionic 1,2,3-triazol-5-ylidene structure. An efficient, cationic palladium catalyst of PEPPSI type, i.e., ''i''PEPPSI (i''nternal'' pyridine-enhanced precatalyst preparation stabilization and initiation) was demonstrated to efficiently catalyse the copper-free Sonogashira reaction in water as the only solvent, under aerobic conditions, in the absence of copper, amines, phosphines and other additives.Metal oxide catalysts
Recent developments in heterogeneous catalysis enabled the use of metal oxide materials such as cuprous oxide nanocatalysts in flow processing technologies, which can enable the economical production of active pharmaceutical ingredients and various other fine chemicals.Applications in synthesis
Sonogashira couplings are employed in a wide array of synthetic reactions, primarily due to their success in facilitating the following challenging transformations:Alkynylation reactions
The coupling of a terminal alkyne and an aromatic ring is the pivotal reaction when talking about applications of the copper-promoted or copper-free Sonogashira reaction. The list of cases where the typical Sonogashira reaction using aryl halides has been employed is large, and choosing illustrative examples is difficult. A recent use of this methodology is shown below for the coupling of iodinated phenylalanine with a terminal alkyne derived from ''d''-biotin using an ''in situ'' generated Pd0 species as catalyst, which allowed the preparation of alkyne-linked phenylalanine derivative for bioanalytical applications. There are also examples of the coupling partners both being attached to allyl resins, with the Pd0 catalyst effecting cleavage of the substrates and subsequent Sonogashira coupling in solution.Natural products
Many metabolites found in nature contain alkyne or enyne moieties, and therefore, the Sonogashira reaction has found frequent utility in their syntheses. Several of the most recent and promising applications of this coupling methodology toward the total synthesis of natural products exclusively employed the typical copper-cocatalyzed reaction. An example of the coupling of an aryl iodide to an aryl acetylene can be seen in the reaction of an iodinated alcohol and tris(isopropyl)silylacetylene, which gave an alkyne, an intermediate in the total synthesis of the benzindenoazepine alkaloid bulgaramine. There are other recent examples of the use of aryl iodides for the preparation of intermediates under typical Sonogashira conditions, which, after cyclization, yield natural products such as benzylisoquinoline or indole alkaloids An example is the synthesis of the benzylisoquinoline alkaloids (+)-(''S'')- laudanosine and (–)-(''S'')-xylopinine. The synthesis of these natural products involved the use of Sonogashira cross-coupling to build the carbon backbone of each molecule.
There are other recent examples of the use of aryl iodides for the preparation of intermediates under typical Sonogashira conditions, which, after cyclization, yield natural products such as benzylisoquinoline or indole alkaloids An example is the synthesis of the benzylisoquinoline alkaloids (+)-(''S'')- laudanosine and (–)-(''S'')-xylopinine. The synthesis of these natural products involved the use of Sonogashira cross-coupling to build the carbon backbone of each molecule.
Enynes and enediynes
The 1,3-enyne moiety is an important structural unit for biologically active and natural compounds. It can be derived from vinylic systems and terminal acetylenes by using a configuration-retention stereospecific procedure such as the Sonogashira reaction. Vinyl iodides are the most reactive vinyl halides to Pd0 oxidative addition, and their use is therefore most frequent for Sonogashira cross-coupling reactions due to the usually milder conditions employed. Some examples include: *The coupling of 2-iodoprop-2-en-1-ol with a wide range of acetylenes. *The preparation of alk-2-ynylbuta-1,3-dienes from the cross-coupling of a diiodide and phenylacetylene, as shown below.
Pharmaceuticals
The versatility of the Sonogashira reaction makes it a widely used reaction in the synthesis of a variety of compounds. One such pharmaceutical application is in the synthesis of SIB-1508Y, which is more commonly known as Altinicline. Altinicline is anicotinic acetylcholine receptor
Nicotinic acetylcholine receptors, or nAChRs, are Receptor (biochemistry), receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the c ...
agonist that has shown potential in the treatment of Parkinson's disease, Alzheimer's disease, Tourette's syndrome, schizophrenia, and attention deficit hyperactivity disorder (ADHD). As of 2008, Altinicline has undergone Phase II clinical trials.

Related reactions
* Cross-coupling reactions ** Castro-Stephens coupling ** Heck reaction ** Stille reaction **Suzuki reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemi ...
** Negishi coupling
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon–carbon bonds (C–C) in the process. A palladium (0) s ...
** Kumada coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically ...
* Transmetalation
References
{{Alkynes Condensation reactions Carbon-carbon bond forming reactions Palladium Organometallic chemistry Name reactions