Seyferth–Gilbert homologation on:
[Wikipedia]
[Google]
[Amazon]
The Seyferth–Gilbert homologation is a
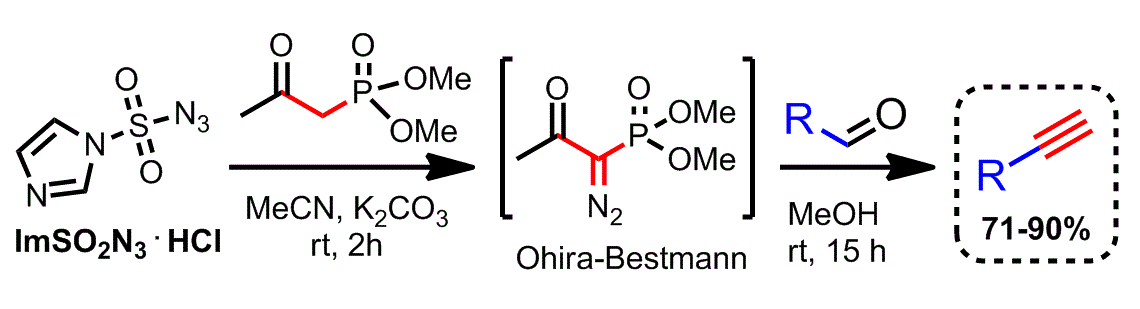 Recently a safer and more scalable approach has been developed for the synthesis of alkynes from aldehydes. This protocol takes advantage of a stable sulfonyl azide, rather than tosyl azide, for the ''in situ'' generation of the Ohira−Bestmann reagent.
Recently a safer and more scalable approach has been developed for the synthesis of alkynes from aldehydes. This protocol takes advantage of a stable sulfonyl azide, rather than tosyl azide, for the ''in situ'' generation of the Ohira−Bestmann reagent.
chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
of an aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
1 (or aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
) with dimethyl (diazomethyl)phosphonate 2 and potassium tert-butoxide
Potassium ''tert''-butoxide (or potassium ''t''-butoxide) is a chemical compound with the formula CH3)3COKsub>''n'' (abbr. KOtBu). This colourless solid is a strong base (pKa of conjugate acid is 17 in H2O), which is useful in organic syn ...
to give substituted alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s 3. Dimethyl (diazomethyl)phosphonate 2 is often called the Seyferth–Gilbert reagent.
This reaction is called a ''homologation
Homologation (Greek language, Greek ''homologeo'', ὁμολογέω, "to agree") is the granting of approval by an official authority. This may be a court of law, a government department, or an academic or professional body, any of which would n ...
'' because the product has exactly one additional carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
more than the starting material.
Reaction mechanism
Deprotonation of the Seyferth–Gilbert reagent A gives an anion B, which reacts with the ketone to form theoxaphosphetane
An oxaphosphetane is a molecule containing a four-membered ring with one phosphorus, one oxygen and two carbon atoms. In a 1,2-oxaphosphetane phosphorus is bonded directly to oxygen, whereas a 1,3-oxaphosphetane has the phosphorus and oxygen atom ...
D. Elimination of dimethylphosphate E gives the vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
diazo
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds ...
-intermediate Fa and Fb. The generation of nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
gas gives a vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
G, which via a 1,2-migration
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over l ...
forms the desired alkyne H.
Bestmann modification
The dimethyl (diazomethyl)phosphonate carbanion can be generated ''in situ'' from dimethyl-1-diazo-2-oxopropylphosphonate (also called the Ohira-Bestmann reagent) by reaction withmethanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
and potassium carbonate
Potassium carbonate is the inorganic compound with the formula . It is a white salt, which is soluble in water and forms a strongly alkaline solution. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used ...
as the base by cleavage of the acetyl group
In organic chemistry, an acetyl group is a functional group denoted by the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, an acetyl grou ...
as methyl acetate
Methyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is a flammable liquid with a characteristically pleasant smell reminiscent of some glues and nail polish remo ...
. Reaction of Bestmann's reagent with aldehydes gives terminal alkynes often in very high yield and fewer steps than the Corey–Fuchs reaction
The Corey–Fuchs reaction, also known as the Ramirez–Corey–Fuchs reaction, is a series of chemical reactions designed to transform an aldehyde into an alkyne. The formation of the 1,1-dibromoolefins via phosphine-dibromomethylenes was origin ...
.
The use of the milder potassium carbonate makes this procedure much more compatible with a wide variety of functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s.
Improved ''in situ'' generation of the Ohira-Bestmann reagent
Other modifications
Another modification for less reactive aldehydes is made by replacement of potassium carbonate withcaesium carbonate
Caesium carbonate or cesium carbonate is a chemical compound with the chemical formula . It is white crystalline solid. Caesium carbonate has a high solubility in polar solvents such as water, ethanol and DMF. Its solubility is higher in organic ...
in MeOH and results in a drastic yield increase.
See also
*Corey–Fuchs reaction
The Corey–Fuchs reaction, also known as the Ramirez–Corey–Fuchs reaction, is a series of chemical reactions designed to transform an aldehyde into an alkyne. The formation of the 1,1-dibromoolefins via phosphine-dibromomethylenes was origin ...
*Horner–Wadsworth–Emmons reaction
The Horner–Wadsworth–Emmons (HWE) reaction is a chemical reaction used in organic chemistry of stabilized phosphonate carbanions with aldehydes (or ketones) to produce predominantly E-alkenes.
In 1958, Leopold Horner published a modifie ...
*Wittig reaction
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most o ...
References
{{DEFAULTSORT:Seyferth-Gilbert homologation Carbon-carbon bond forming reactions Rearrangement reactions Name reactions