Schrock Carbene on:
[Wikipedia]
[Google]
[Amazon]
A transition metal carbene complex is an
 The common features of Fischer carbenes are:
* low
The common features of Fischer carbenes are:
* low 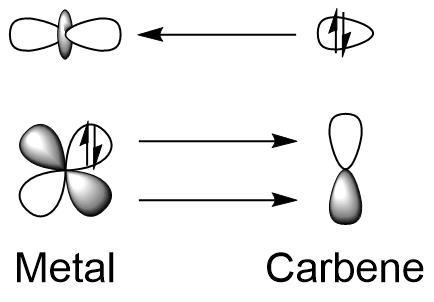 Fischer carbene complexes are related to the singlet form of carbenes, where both electrons occupy the same sp2 orbital at the carbon. This lone pair donates to a metal-based empty d orbital, forming a σ bond. π-backbonding from a filled metal d orbital to the empty p orbital of the carbon atom is possible. However this interaction is generally weak since the alpha donor atoms also donate to this orbital. As such, Fischer carbenes are characterized as having partial double bond character. The major resonance structures of Fischer carbenes put the negative charge on the metal centre, and the positive on the carbon atom, making it electrophilic.
Fischer carbene complexes are related to the singlet form of carbenes, where both electrons occupy the same sp2 orbital at the carbon. This lone pair donates to a metal-based empty d orbital, forming a σ bond. π-backbonding from a filled metal d orbital to the empty p orbital of the carbon atom is possible. However this interaction is generally weak since the alpha donor atoms also donate to this orbital. As such, Fischer carbenes are characterized as having partial double bond character. The major resonance structures of Fischer carbenes put the negative charge on the metal centre, and the positive on the carbon atom, making it electrophilic.
 Fischer carbenes can be likened to ketones, with the carbene carbon atom being electrophilic, like the carbonyl carbon atom of a ketone. This can be seen from the
Fischer carbenes can be likened to ketones, with the carbene carbon atom being electrophilic, like the carbonyl carbon atom of a ketone. This can be seen from the
 Schrock carbenes do not have π-accepting ligands on the metal centre. They are often called alkylidene complexes. Typically this subset of carbene complexes are found with:
* high
Schrock carbenes do not have π-accepting ligands on the metal centre. They are often called alkylidene complexes. Typically this subset of carbene complexes are found with:
* high 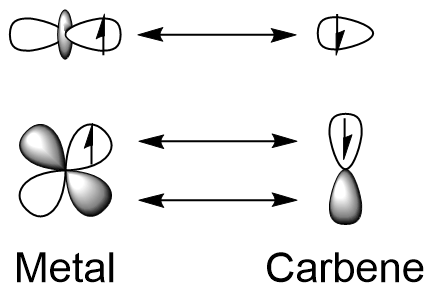 Bonding in such complexes can be viewed as the coupling of a triplet state metal and triplet carbene, forming a true double bond. Both the metal and carbon atom donate 2 electrons, one to each bond. Since there is no donation to the carbene atom from adjacent groups, the extent of
Bonding in such complexes can be viewed as the coupling of a triplet state metal and triplet carbene, forming a true double bond. Both the metal and carbon atom donate 2 electrons, one to each bond. Since there is no donation to the carbene atom from adjacent groups, the extent of 
 The dominant application of metal carbenes involves none of the above classes of compounds, but rather
The dominant application of metal carbenes involves none of the above classes of compounds, but rather
 Diazo compounds like
Diazo compounds like 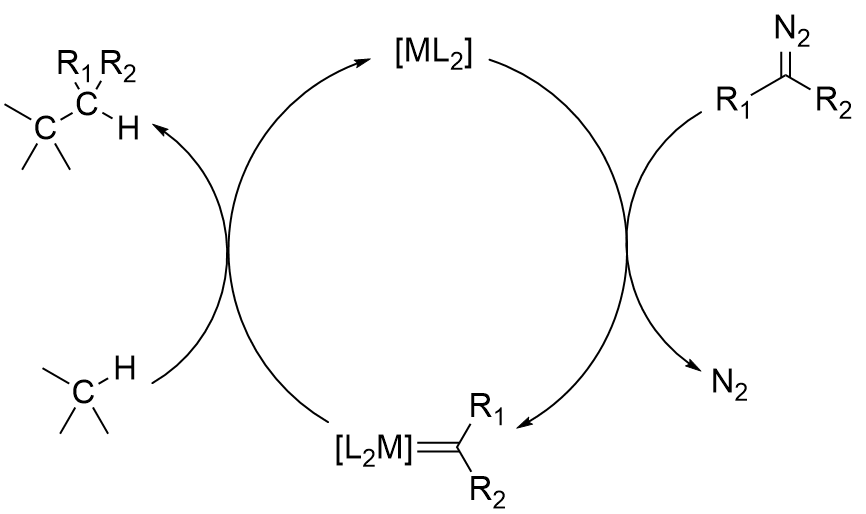
organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and ...
featuring a divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemica ...
carbon ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
, itself also called a carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
. Carbene complexes have been synthesized from most transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s and f-block metals, using many different synthetic routes such as nucleophilic addition and alpha-hydrogen abstraction. The term carbene ligand is a formalism since many are not directly derived from carbenes and most are much less reactive than lone carbenes. Described often as , carbene ligands are intermediate between alkyls and carbynes . Many different carbene-based reagents such as Tebbe's reagent
Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylidenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. ...
are used in synthesis. They also feature in catalytic reactions, especially alkene metathesis, and are of value in both industrial heterogeneous and in homogeneous catalysis for laboratory- and industrial-scale preparation of fine chemicals.
Classification
Metal carbene complexes are often classified into two types. The Fischer carbenes, named after Ernst Otto Fischer, feature strong π-acceptors at the metal and areelectrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
at the carbene carbon atom. Schrock carbenes, named after Richard R. Schrock, are characterized by more nucleophilic carbene carbon centers; these species typically feature higher oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
(valency) metals. ''N''-Heterocyclic carbenes (NHCs) were popularized following Arduengo's isolation of a stable free carbene in 1991. Reflecting the growth of the area, carbene complexes are now known with a broad range of different reactivities and diverse substituents. Often it is not possible to classify a carbene complex solely with regards to its electrophilicity or nucleophilicity.
Fischer carbenes
 The common features of Fischer carbenes are:
* low
The common features of Fischer carbenes are:
* low oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
metal center
* middle and late transition metals Fe(0), Mo(0), Cr(0)
* π-acceptor metal ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s
* π-donor substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
s on the carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
atom such as alkoxy
In chemistry, the alkoxy group is an alkyl group which is Single bond, singularly bonded to oxygen; thus . Denoted usually with apostrophe('). The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the ...
and alkylated amino
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
groups.
Examples include and .
 Fischer carbene complexes are related to the singlet form of carbenes, where both electrons occupy the same sp2 orbital at the carbon. This lone pair donates to a metal-based empty d orbital, forming a σ bond. π-backbonding from a filled metal d orbital to the empty p orbital of the carbon atom is possible. However this interaction is generally weak since the alpha donor atoms also donate to this orbital. As such, Fischer carbenes are characterized as having partial double bond character. The major resonance structures of Fischer carbenes put the negative charge on the metal centre, and the positive on the carbon atom, making it electrophilic.
Fischer carbene complexes are related to the singlet form of carbenes, where both electrons occupy the same sp2 orbital at the carbon. This lone pair donates to a metal-based empty d orbital, forming a σ bond. π-backbonding from a filled metal d orbital to the empty p orbital of the carbon atom is possible. However this interaction is generally weak since the alpha donor atoms also donate to this orbital. As such, Fischer carbenes are characterized as having partial double bond character. The major resonance structures of Fischer carbenes put the negative charge on the metal centre, and the positive on the carbon atom, making it electrophilic.
 Fischer carbenes can be likened to ketones, with the carbene carbon atom being electrophilic, like the carbonyl carbon atom of a ketone. This can be seen from the
Fischer carbenes can be likened to ketones, with the carbene carbon atom being electrophilic, like the carbonyl carbon atom of a ketone. This can be seen from the resonance structures
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
, where there is a significant contribution from the structure bearing a positive carbon centre. Like ketones, Fischer carbene species can undergo aldol
In organic chemistry, an aldol is a structure consisting of a hydroxy group (-OH) two carbons away from either an aldehyde or a ketone. The name combines the suffix 'ol' from the alcohol and the prefix depending on the carbonyl group, either 'ald' ...
-like reactions. The hydrogen atoms attached to the carbon atom α to the carbene carbon atom are acidic, and can be deprotonated by a base such as ''n''-butyllithium, to give a nucleophile, which can undergo further reaction.
Schrock carbenes
 Schrock carbenes do not have π-accepting ligands on the metal centre. They are often called alkylidene complexes. Typically this subset of carbene complexes are found with:
* high
Schrock carbenes do not have π-accepting ligands on the metal centre. They are often called alkylidene complexes. Typically this subset of carbene complexes are found with:
* high oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
metal center
* early transition metals Ti(IV), Ta(V)
* σ-donor and sometimes π-donor metal ligands
* hydrogen and alkyl substituents on carbenoid carbon.
Examples include and .
 Bonding in such complexes can be viewed as the coupling of a triplet state metal and triplet carbene, forming a true double bond. Both the metal and carbon atom donate 2 electrons, one to each bond. Since there is no donation to the carbene atom from adjacent groups, the extent of
Bonding in such complexes can be viewed as the coupling of a triplet state metal and triplet carbene, forming a true double bond. Both the metal and carbon atom donate 2 electrons, one to each bond. Since there is no donation to the carbene atom from adjacent groups, the extent of pi backbonding
In chemistry, pi backbonding or π backbonding is a π-bonding interaction between a filled (or half filled) orbital of a transition metal atom and a vacant orbital on an adjacent ion or molecule. In this type of interaction, electrons from the ...
is much greater, giving a strong double bond. These bonds are weakly polarized towards carbon and therefore the carbene atom is a nucleophile. Furthermore, the major resonance structures of Schrock carbene put the negative charge on the carbon atom, making it nucleophilic. Complexes with the methylidene
Methylene (IUPAC name: methylidene, also called carbene or methene) is an organic compound with the chemical formula (also written and not to be confused with compressed hydrogen, which is also denoted ). It is a colourless gas that fluoresces ...
ligand () are the simplest Schrock-type carbenes.

''N''-Heterocyclic carbenes
''N''-Heterocyclic carbenes (NHCs) are particularly common carbene ligands. They are popular because they are more readily prepared than Schrock and Fischer carbenes. In fact, many NHCs are isolated as the free ligand, since they arepersistent carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with octet rule, incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with ...
s. Being strongly stabilized by π-donating substituents, NHCs are powerful σ-donors but π-bonding with the metal is weak. For this reason, the bond between the carbon and the metal center is often represented by a single dative bond, whereas Fischer and Schrock carbenes are usually depicted with double bonds to metal. Continuing with this analogy, NHCs are often compared with trialkylphosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
ligands. Like phosphines, NHCs serve as spectator ligand In coordination chemistry, a spectator ligand is a ligand that does not participate in chemical reactions of the complex. Instead, spectator ligands (vs "actor ligands") occupy coordination sites. Spectator ligands tend to be of polydentate, such th ...
s that influence catalysis through a combination of electronic and steric effects, but they do not directly bind substrates. Examples to NHC complexes of transition metals include coinage metal NHC complexes, and cyclic iron tetra N-heterocyclic carbenes.
Bimetallic carbene complexes
An early example of this bonding mode was provided by prepared fromdiazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow ga ...
:
:
Another example of this family of compounds is Tebbe's reagent
Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylidenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. ...
. It features a methylene bridge joining titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
and aluminum
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
.
Application of Metal Carbenes
Metal carbene complexes have applications in hetereogeneous and homogeneous catalysis, and as reagents for organic reactions.Catalysis
 The dominant application of metal carbenes involves none of the above classes of compounds, but rather
The dominant application of metal carbenes involves none of the above classes of compounds, but rather heterogeneous catalyst
Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reagents or products. The process contrasts with homogeneous catalysis where the reagents, products and catalyst exist in the same phase. Phase distingui ...
s used for alkene metathesis for the synthesis of higher alkenes. A variety of related reactions are used to interconvert light alkenes, e.g. butenes, propylene, and ethylene. Carbene complexes are invoked as intermediates in the Fischer–Tropsch route to hydrocarbons.
A variety of homogeneous carbene catalysts, especially the Grubbs' ruthenium and Schrock molybdenum-imido catalysts have been used for olefin metathesis in laboratory-scale synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organi ...
of natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
s and materials science
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries.
The intellectual origins of materials sci ...
.
Stoichiometric reactions
Homogeneous Schrock-type carbene complexes such asTebbe's reagent
Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylidenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. ...
can be used for the olefination of carbonyls, replacing the oxygen atom with a methylidene group. The nucleophilic carbon atom behaves similarly to the carbon atom of the phosphorus ylide in the Wittig reaction
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most o ...
, attacking the electrophilic carbonyl atom of a ketone, followed by elimination of a metal oxide.
In the nucleophilic abstraction
Nucleophilic abstraction is a type of an organometallic reaction which can be defined as a nucleophilic attack on a ligand which causes part or all of the original ligand to be removed from the metal along with the nucleophile.Spessard, Gary; Miess ...
reaction, a methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated a ...
can be abstracted from the donating group of a Fischer carbene, making it a strong nucleophile for further reaction.
 Diazo compounds like
Diazo compounds like methyl phenyldiazoacetate
Methyl phenyldiazoacetate is the organic compound with the formula C6H5C(N2)CO2Me. It is a diazo derivative of methyl phenylacetate. Colloquially referred to as "phenyldiazoacetate", it is generated and used ''in situ
is a Latin phrase mea ...
can be used for cyclopropanation or to insert into C-H bonds of organic substrates. These reactions are catalyzed by dirhodium tetraacetate
Rhodium(II) acetate is the coordination compound with the formula Rh2(AcO)4, where AcO− is the acetate ion (). This dark green powder is slightly soluble in polar solvents, including water. It is used as a catalyst for cyclopropanation of alke ...
or related chiral derivatives. Such catalysis is assumed to proceed via the intermediacy of carbene complexes.

Wulff-Dötz Reaction
Fischer carbenes are used with alkynes as the starting reagents for the Wulff–Dötz reaction, forming phenols.History
The first metal carbene complex to have been reported was Chugaev's red salt, first synthesized as early as 1925, although it was never identified to be a carbene complex. The characterization of (CO)5W(COCH3(Ph)) in the 1960s is often cited as the starting point of the area and Ernst Otto Fischer, for this and other achievements in organometallic chemistry, was awarded the 1973Nobel Prize in Chemistry
The Nobel Prize in Chemistry () is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outst ...
. In 1968, Hans-Werner Wanzlick
Hans-Werner Wanzlick (1917-1988) was a German chemist. A Professor of chemistry at Technische Universität Berlin he is notable for work on persistent carbenes and for proposing the Wanzlick equilibrium between saturated imidazolin-2-ylidenes a ...
and Karl Öfele separately reported metal-bonded N-heterocyclic carbenes. The synthesis and characterization of ((CH3)3CCH2)Ta=CHC(CH3)3 by Richard R. Schrock in 1974 marked the first metal alkylidene complex. In 1991, Anthony J. Arduengo synthesized and crystallized the first persistent carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with octet rule, incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with ...
, an NHC with large adamantane
Adamantane is an organic compound with formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the mo ...
alkyl groups, accelerating the field of N-heterocarbene ligands to its current use.
See also
*Carbene radical
Carbene radicals are a special class of organometallic carbenes. The carbene radical can be formed by one-electron reduction of Fischer-type carbenes using an external reducing agent, or directly upon carbene formation at an open-shell transitio ...
* Carbyne
In organic chemistry, a carbyne is a general term for any compound whose structure consists of an electrically neutral carbon atom connected by a single covalent bond and has three non-bonded electrons. The carbon atom has either one or three ...
* Transition metal carbyne complex
* Transition metal vinylidene complex
A transition metal vinylidene complex is an organometallic compound containing a metal bound to a vinylidene group (i.e. bearing the motif M=C=CRR'). Free Vinylidene group, vinylidenes (:C=CRR') are the less thermodynamically stable valence taut ...
References
{{Coordination complexes Organometallic chemistry Carbenes Transition metals Coordination chemistry