Saturation pressure on:
[Wikipedia]
[Google]
[Amazon]

 Vapor pressure or equilibrium vapor pressure is the
Vapor pressure or equilibrium vapor pressure is the
(Chemistry Department,
 As a general trend, vapor pressures of liquids at ambient temperatures increase with decreasing boiling points. This is illustrated in the vapor pressure chart (see right) that shows graphs of the vapor pressures versus temperatures for a variety of liquids. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, 760Torr, 101.325kPa, or 14.69595psi.
For example, at any given temperature,
As a general trend, vapor pressures of liquids at ambient temperatures increase with decreasing boiling points. This is illustrated in the vapor pressure chart (see right) that shows graphs of the vapor pressures versus temperatures for a variety of liquids. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, 760Torr, 101.325kPa, or 14.69595psi.
For example, at any given temperature,
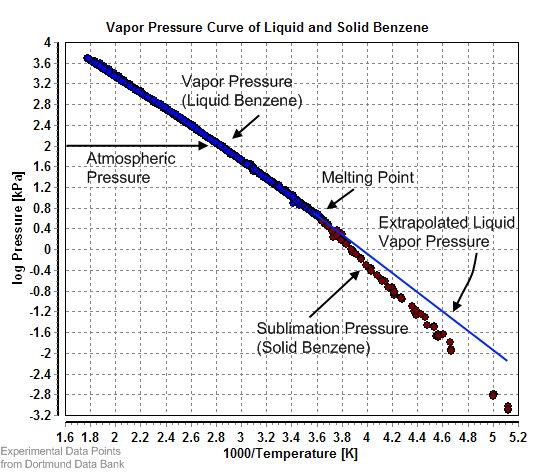 Equilibrium vapor pressure can be defined as the pressure reached when a condensed phase is in equilibrium with its own vapor. In the case of an equilibrium solid, such as a
Equilibrium vapor pressure can be defined as the pressure reached when a condensed phase is in equilibrium with its own vapor. In the case of an equilibrium solid, such as a
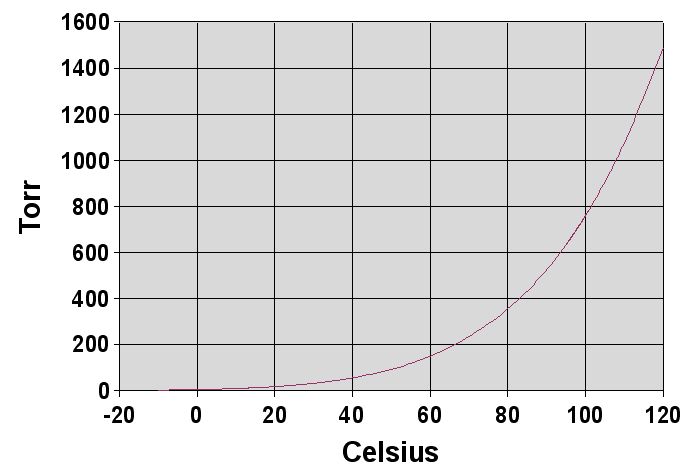 Like all liquids, water boils when its vapor pressure reaches its surrounding pressure. In nature, the atmospheric pressure is lower at higher elevations and water boils at a lower temperature. The boiling temperature of water for atmospheric pressures can be approximated by the
Like all liquids, water boils when its vapor pressure reaches its surrounding pressure. In nature, the atmospheric pressure is lower at higher elevations and water boils at a lower temperature. The boiling temperature of water for atmospheric pressures can be approximated by the
Fluid Characteristics Chart
Engineer's Edge
Hyperphysics
The MSDS HyperGlossary
Online vapor pressure calculation tool (Requires Registration)Prediction of Vapor Pressures of Pure Liquid Organic Compounds
{{Authority control Engineering thermodynamics Gases Meteorological concepts Meteorological quantities Pressure Thermodynamic properties
 Vapor pressure or equilibrium vapor pressure is the
Vapor pressure or equilibrium vapor pressure is the pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
exerted by a vapor
In physics, a vapor (American English) or vapour (Commonwealth English; American and British English spelling differences#-our, -or, see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R ...
in thermodynamic equilibrium
Thermodynamic equilibrium is a notion of thermodynamics with axiomatic status referring to an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable ...
with its condensed phases (solid or liquid) at a given temperature in a closed system
A closed system is a natural physical system that does not allow transfer of matter in or out of the system, althoughin the contexts of physics, chemistry, engineering, etc.the transfer of energy (e.g. as work or heat) is allowed.
Physics
In cl ...
. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid (or solid) in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as '' volatile''. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions.
The vapor pressure of any substance increases non-linearly with temperature, often described by the Clausius–Clapeyron relation
The Clausius–Clapeyron relation, in chemical thermodynamics, specifies the temperature dependence of pressure, most importantly vapor pressure, at a discontinuous phase transition between two phases of matter of a single constituent. It is nam ...
. The atmospheric pressure
Atmospheric pressure, also known as air pressure or barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1,013. ...
boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of a liquid (also known as the normal boiling point) is the temperature at which the vapor pressure equals the ambient atmospheric pressure. With any incremental increase in that temperature, the vapor pressure becomes sufficient to overcome atmospheric pressure
Atmospheric pressure, also known as air pressure or barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1,013. ...
and cause the liquid to form vapor bubbles. Bubble
Bubble, Bubbles or The Bubble may refer to:
Common uses
* Bubble (physics), a globule of one substance in another, usually gas in a liquid
** Soap bubble
* Economic bubble, a situation where asset prices are much higher than underlying fundame ...
formation in greater depths of liquid requires a slightly higher temperature due to the higher fluid pressure, due to hydrostatic pressure of the fluid mass above. More important at shallow depths is the higher temperature required to start bubble formation. The surface tension of the bubble wall leads to an overpressure in the very small initial bubbles.
Measurement and units
Vapor pressure is measured in the standard units ofpressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
. The International System of Units
The International System of Units, internationally known by the abbreviation SI (from French ), is the modern form of the metric system and the world's most widely used system of measurement. It is the only system of measurement with official s ...
(SI) recognizes pressure as a derived unit
A base unit of measurement (also referred to as a base unit or fundamental unit) is a unit of measurement adopted for a '' base quantity''. A base quantity is one of a conventionally chosen subset of physical quantities, where no quantity in the ...
with the dimension of force per area and designates the pascal (Pa) as its standard unit. One pascal is one newton per square meter
The square metre ( international spelling as used by the International Bureau of Weights and Measures) or square meter (American spelling) is the unit of area in the International System of Units (SI) with symbol m2. It is the area of a square w ...
(N·m−2 or kg·m−1·s−2).
Experimental measurement of vapor pressure is a simple procedure for common pressures between 1 and 200 kPa. The most accurate results are obtained near the boiling point of the substance; measurements smaller than are subject to major errors. Procedures often consist of purifying the test substance, isolating it in a container, evacuating any foreign gas, then measuring the equilibrium pressure of the gaseous phase of the substance in the container at different temperatures. Better accuracy is achieved when care is taken to ensure that the entire substance and its vapor are both at the prescribed temperature. This is often done, as with the use of an isoteniscope, by submerging the containment area in a liquid bath.
Very low vapor pressures of solids can be measured using the Knudsen effusion cell
In crystal growth, a Knudsen cell is an effusion evaporator source for relatively low partial pressure elementary sources (e.g. Ga, Al, Hg, As). Because it is easy to control the temperature of the evaporating material in Knudsen cells, they are ...
method.
In a medical context, vapor pressure is sometimes expressed in other units, specifically millimeters of mercury (mmHg). Accurate knowledge of the vapor pressure is important for volatile inhalational anesthetic
An inhalational anesthetic is a chemical compound possessing general anesthetic properties that is delivered via inhalation. They are administered through a face mask, laryngeal mask airway or tracheal tube connected to an anesthetic vaporiser ...
s, most of which are liquids at body temperature but have a relatively high vapor pressure.
Estimating vapor pressures with Antoine equation
TheAntoine equation
The Antoine equation is a class of semi-empirical correlations describing the relation between vapor pressure and temperature for pure substances. The Antoine equation is derived from the Clausius–Clapeyron relation. The equation was presente ...
What is the Antoine Equation?(Chemistry Department,
Frostburg State University
Frostburg State University (FSU) is a public university in Frostburg, Maryland. The university is the only four-year institution of the University System of Maryland west of the Baltimore-Washington passageway in the state's Appalachian highlan ...
, Maryland
Maryland ( ) is a U.S. state, state in the Mid-Atlantic (United States), Mid-Atlantic region of the United States. It borders the states of Virginia to its south, West Virginia to its west, Pennsylvania to its north, and Delaware to its east ...
) is a pragmatic mathematical expression of the relation between the vapor pressure and the temperature of pure liquid or solid substances. It is obtained by curve-fitting and is adapted to the fact that vapor pressure is usually increasing and concave as a function of temperature. The basic form of the equation is:
:
and it can be transformed into this temperature-explicit form:
:
where:
* is the absolute vapor pressure of a substance
* is the temperature of the substance
* , and are substance-specific coefficients (i.e., constants or parameters)
* is typically either or
A simpler form of the equation with only two coefficients is sometimes used:
:
which can be transformed to:
:
Sublimations and vaporizations of the same substance have separate sets of Antoine coefficients, as do components in mixtures. Each parameter set for a specific compound is only applicable over a specified temperature range. Generally, temperature ranges are chosen to maintain the equation's accuracy of a few up to 8–10 percent. For many volatile substances, several different sets of parameters are available and used for different temperature ranges. The Antoine equation has poor accuracy with any single parameter set when used from a compound's melting point to its critical temperature. Accuracy is also usually poor when vapor pressure is under 10 Torr because of the limitations of the apparatus used to establish the Antoine parameter values.
The Wagner equation gives "one of the best" fits to experimental data but is quite complex. It expresses reduced vapor pressure as a function of reduced temperature.
Relation to boiling point of liquids
methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in indus ...
has the highest vapor pressure of any of the liquids in the chart. It also has the lowest normal boiling point at , which is where the vapor pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere ( atm) of absolute vapor pressure.
Although the relation between vapor pressure and temperature is non-linear, the chart uses a logarithmic vertical axis to produce slightly curved lines, so one chart can graph many liquids. A nearly straight line is obtained when the logarithm of the vapor pressure is plotted against 1/(T + 230) where T is the temperature in degrees Celsius. The vapor pressure of a liquid at its boiling point equals the pressure of its surrounding environment.
Liquid mixtures: Raoult's law
Raoult's law
Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is ...
gives an approximation to the vapor pressure of mixtures of liquids. It states that the activity (pressure or fugacity
In thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of chemical equilibrium. It is equal to the pressure of an ideal gas which has the same tempe ...
) of a single-phase mixture is equal to the mole-fraction-weighted sum of the components' vapor pressures:
:
where is the mixture's vapor pressure, is the mole fraction
In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount of a constituent substance, ''ni'' (expressed in unit of moles, symbol mol), and the to ...
of component in the liquid phase and is the mole fraction
In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount of a constituent substance, ''ni'' (expressed in unit of moles, symbol mol), and the to ...
of component in the vapor phase respectively. is the vapor pressure of component . Raoult's law is applicable only to non-electrolytes (uncharged species); it is most appropriate for non-polar molecules with only weak intermolecular attractions (such as London forces).
Systems that have vapor pressures higher than indicated by the above formula are said to have positive deviations. Such a deviation suggests weaker intermolecular attraction than in the pure components, so that the molecules can be thought of as being "held in" the liquid phase less strongly than in the pure liquid. An example is the azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens beca ...
of approximately 95% ethanol and water. Because the azeotrope's vapor pressure is higher than predicted by Raoult's law, it boils at a temperature below that of either pure component.
There are also systems with negative deviations that have vapor pressures that are lower than expected. Such a deviation is evidence for stronger intermolecular attraction between the constituents of the mixture than exists in the pure components. Thus, the molecules are "held in" the liquid more strongly when a second molecule is present. An example is a mixture of trichloromethane (chloroform) and 2-propanone (acetone), which boils above the boiling point of either pure component.
The negative and positive deviations can be used to determine thermodynamic activity
In thermodynamics, activity (symbol ) is a measure of the "effective concentration" of a species in a mixture, in the sense that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on conc ...
coefficients of the components of mixtures.
Solids
 Equilibrium vapor pressure can be defined as the pressure reached when a condensed phase is in equilibrium with its own vapor. In the case of an equilibrium solid, such as a
Equilibrium vapor pressure can be defined as the pressure reached when a condensed phase is in equilibrium with its own vapor. In the case of an equilibrium solid, such as a crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
, this can be defined as the pressure when the rate of sublimation of a solid matches the rate of deposition of its vapor phase. For most solids this pressure is very low, but some notable exceptions are naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation ...
, dry ice
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and Sublimation (phase transition), sublimes directly from the solid state to the gas ...
(the vapor pressure of dry ice is 5.73 MPa (831 psi, 56.5 atm) at 20 °C, which causes most sealed containers to rupture), and ice. All solid materials have a vapor pressure. However, due to their often extremely low values, measurement can be rather difficult. Typical techniques include the use of thermogravimetry and gas transpiration.
There are a number of methods for calculating the sublimation pressure (i.e., the vapor pressure) of a solid. One method is to estimate the sublimation pressure from extrapolated liquid vapor pressures (of the supercooled liquid), if the heat of fusion
In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing energy, typically heat, to a specific quantity of the substance to change its state from a s ...
is known, by using this particular form of the Clausius–Clapeyron relation:
:
where:
* is the sublimation pressure of the solid component at the temperature .
* is the extrapolated vapor pressure of the liquid component at the temperature .
* is the heat of fusion.
* is the gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment p ...
.
* is the sublimation temperature.
* is the melting point temperature.
This method assumes that the heat of fusion is temperature-independent, ignores additional transition temperatures between different solid phases, and it gives a fair estimation for temperatures not too far from the melting point. It also shows that the sublimation pressure is lower than the extrapolated liquid vapor pressure (Δfus''H'' > 0) and the difference grows with increased distance from the melting point.
Boiling point of water
 Like all liquids, water boils when its vapor pressure reaches its surrounding pressure. In nature, the atmospheric pressure is lower at higher elevations and water boils at a lower temperature. The boiling temperature of water for atmospheric pressures can be approximated by the
Like all liquids, water boils when its vapor pressure reaches its surrounding pressure. In nature, the atmospheric pressure is lower at higher elevations and water boils at a lower temperature. The boiling temperature of water for atmospheric pressures can be approximated by the Antoine equation
The Antoine equation is a class of semi-empirical correlations describing the relation between vapor pressure and temperature for pure substances. The Antoine equation is derived from the Clausius–Clapeyron relation. The equation was presente ...
:
:
or transformed into this temperature-explicit form:
:
where the temperature is the boiling point in degrees Celsius
The degree Celsius is the unit of temperature on the Celsius temperature scale "Celsius temperature scale, also called centigrade temperature scale, scale based on 0 ° for the melting point of water and 100 ° for the boiling point ...
and the pressure is in torr
The torr (symbol: Torr) is a Pressure#Units, unit of pressure based on an absolute scale, defined as exactly of a standard atmosphere (unit), atmosphere (101325 Pa). Thus one torr is exactly (≈ ).
Historically, one torr was intended to be ...
.
Dühring's rule
Dühring's rule states that a linear relationship exists between the temperatures at which two solutions exert the same vapor pressure.Examples
The following table is a list of a variety of substances ordered by increasing vapor pressure (in absolute units).Estimating vapor pressure from molecular structure
Several empirical methods exist to estimate the vapor pressure from molecular structure for organic molecules. Some examples are SIMPOL.1 method, the method of Moller et al., and EVAPORATION (Estimation of VApour Pressure of ORganics, Accounting for Temperature, Intramolecular, and Non-additivity effects).Meaning in meteorology
Inmeteorology
Meteorology is the scientific study of the Earth's atmosphere and short-term atmospheric phenomena (i.e. weather), with a focus on weather forecasting. It has applications in the military, aviation, energy production, transport, agricultur ...
, the term vapor pressure means the partial pressure of water vapor in the atmosphere, even if it is not in equilibrium.
This differs from its meaning in other sciences.
According to the American Meteorological Society
The American Meteorological Society (AMS) is a scientific and professional organization in the United States promoting and disseminating information about the atmospheric, oceanic, and hydrologic sciences. Its mission is to advance the atmosph ...
''Glossary of Meteorology'', saturation vapor pressure properly refers to the equilibrium vapor pressure of water above a flat surface of liquid water or solid ice, and is a function only of temperature and whether the condensed phase is liquid or solid.
Relative humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation (meteorology), precipitation, dew, or fog t ...
is defined relative to saturation vapor pressure.
Equilibrium vapor pressure does not require the condensed phase to be a flat surface; it might consist of tiny droplets possibly containing solutes (impurities), such as a cloud
In meteorology, a cloud is an aerosol consisting of a visible mass of miniature liquid droplets, frozen crystals, or other particles, suspended in the atmosphere of a planetary body or similar space. Water or various other chemicals may ...
. Equilibrium vapor pressure may differ significantly from saturation vapor pressure depending on the size of droplets and presence of other particles which act as cloud condensation nuclei.
However, these terms are used inconsistently, and some authors use ''"saturation vapor pressure"'' outside the narrow meaning given by the AMS ''Glossary''. For example, a text on atmospheric convection
Atmospheric convection is the vertical transport of heat and moisture in the atmosphere. It occurs when warmer, less dense air rises, while cooler, denser air sinks.
This process is driven by parcel-environment instability, meaning that a "par ...
states, "The Kelvin effect causes the saturation vapor pressure over the curved surface of the droplet to be greater than that over a flat water surface" (emphasis added).
The still-current term ''saturation vapor pressure'' derives from the obsolete theory that water vapor dissolves into air, and that air at a given temperature can only hold a certain amount of water before becoming "saturated". Actually, as stated by Dalton's law (known since 1802), the partial pressure of water vapor or any substance does not depend on air at all, and the relevant temperature is that of the liquid. Nevertheless, the erroneous belief persists among the public and even meteorologists, aided by the misleading terms ''saturation pressure'' and ''supersaturation'' and the related definition of ''relative humidity''.
(Alternate title: "Water Vapor Myths: A Brief Tutorial".)
See also
* Absolute humidity *Antoine equation
The Antoine equation is a class of semi-empirical correlations describing the relation between vapor pressure and temperature for pure substances. The Antoine equation is derived from the Clausius–Clapeyron relation. The equation was presente ...
* Lee–Kesler method
The Lee–Kesler method
allows the estimation of the saturated vapor pressure at a given temperature for all components for which the critical pressure ''P''c, the critical temperature ''T''c, and the acentric factor ''ω'' are known.
Equati ...
* Osmotic coefficient
An osmotic coefficient \phi is a quantity which characterises the deviation of a solvent from ideal behaviour, referenced to Raoult's law. It can be also applied to solutes. Its definition depends on the ways of expressing chemical composition
A ...
* Raoult's law
Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is ...
: vapor pressure lowering in solution
* Reid vapor pressure {{Short description, Measure of the volatility of gasoline and other petroleum products
Reid vapor pressure (RVP) is a common measure of the Gasoline#Volatility, volatility of gasoline and other petroleum products. It is defined as the
absolute va ...
* Relative humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation (meteorology), precipitation, dew, or fog t ...
* Relative volatility
Relative volatility is a measure comparing the vapor pressures of the components in a liquid mixture of chemicals. This quantity is widely used in designing large industrial distillation processes. In effect, it indicates the ease or difficulty of ...
* Saturation vapor density
* Triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at ...
* True vapor pressure
* Vapor–liquid equilibrium
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase.
The Vapor quality, concentration of a vapor in contact with its liquid, ...
* Vapor pressures of the elements (data page)
* Vapour pressure of water
The vapor pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as air). The saturation vapor pressure is the pressure at which water vapor is in thermodynamic eq ...
* High-pressure chemistry
Notes
References
External links
Fluid Characteristics Chart
Engineer's Edge
Hyperphysics
The MSDS HyperGlossary
Online vapor pressure calculation tool (Requires Registration)
{{Authority control Engineering thermodynamics Gases Meteorological concepts Meteorological quantities Pressure Thermodynamic properties