Saquinavir on:
[Wikipedia]
[Google]
[Amazon]
Saquinavir, sold under the brand name Invirase among others, is an
 Saquinavir was developed by the pharmaceutical company
Saquinavir was developed by the pharmaceutical company
antiretroviral medication
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs as a strategy to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of mul ...
used together with other medications to treat or prevent HIV/AIDS
The HIV, human immunodeficiency virus (HIV) is a retrovirus that attacks the immune system. Without treatment, it can lead to a spectrum of conditions including acquired immunodeficiency syndrome (AIDS). It is a Preventive healthcare, pr ...
. Typically it is used with ritonavir
Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Ritonavir is a protease inhi ...
or lopinavir/ritonavir
Lopinavir/ritonavir (LPV/r), sold under the brand name Kaletra among others, is a fixed-dose combination antiretroviral medication for the treatment and prevention of HIV/AIDS. It combines lopinavir with a low dose of ritonavir. It is gene ...
to increase its effect. It is taken by mouth
Oral administration is a route of administration whereby a substance is taken through the Human mouth, mouth, swallowed, and then processed via the digestive system. This is a common route of administration for many medications.
Oral administ ...
.
Common side effects include nausea, vomiting, diarrhea, and feeling tired. More serious side effects include problems with QT prolongation, heart block, high blood lipids, and liver problems. It appears to be safe in pregnancy. It is in the protease inhibitor class and works by blocking the HIV protease
The human immunodeficiency viruses (HIV) are two species of '' Lentivirus'' (a subgroup of retrovirus) that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the im ...
.
Saquinavir was patented in 1988 and first sold in 1995.
Medical uses
Saquinavir is used together with other medications to treat or preventHIV/AIDS
The HIV, human immunodeficiency virus (HIV) is a retrovirus that attacks the immune system. Without treatment, it can lead to a spectrum of conditions including acquired immunodeficiency syndrome (AIDS). It is a Preventive healthcare, pr ...
. Typically it is used with ritonavir
Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Ritonavir is a protease inhi ...
or lopinavir/ritonavir
Lopinavir/ritonavir (LPV/r), sold under the brand name Kaletra among others, is a fixed-dose combination antiretroviral medication for the treatment and prevention of HIV/AIDS. It combines lopinavir with a low dose of ritonavir. It is gene ...
to increase its effect.
Side effects
The most frequent adverse events with saquinavir in either formulation are mild gastrointestinal symptoms, includingdiarrhoea
Diarrhea (American English), also spelled diarrhoea or diarrhœa (British English), is the condition of having at least three loose, liquid, or watery bowel movements in a day. It often lasts for a few days and can result in dehydration d ...
, nausea
Nausea is a diffuse sensation of unease and discomfort, sometimes perceived as an urge to vomit. It can be a debilitating symptom if prolonged and has been described as placing discomfort on the chest, abdomen, or back of the throat.
Over 30 d ...
, loose stools and abdominal discomfort. Invirase is better tolerated than Fortovase.
Bioavailability and drug interactions
Saquinavir, in the Invirase formulation, has a low and variable oral bioavailability, when given alone. The Fortovase formulation at the standard dosage delivers approximately eightfold more active drug than Invirase, also at the standard dosage. In the clinic, it was found that the oral bioavailability of saquinavir in both formulations significantly increases when patients also receive the PIritonavir
Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Ritonavir is a protease inhi ...
. For patients, this has the major benefit that they can take less saquinavir, while maintaining sufficient saquinavir blood plasma levels to efficiently suppress the replication of HIV.
The mechanism behind this welcome observation was not directly known, but later it was determined that ritonavir inhibits the cytochrome P450
Cytochromes P450 (P450s or CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for examp ...
3A4 isozyme. Normally, this enzyme metabolizes saquinavir to an inactive form, but with the ritonavir inhibiting this enzyme, the saquinavir blood plasma levels increased considerably. Additionally, ritonavir also inhibits multidrug transporters, although to a much lower extent.
Unlike other protease inhibitors, the absorption of saquinavir seems to be improved by omeprazole
Omeprazole, sold under the brand names Prilosec and Losec, among others, is a medication used in the treatment of gastroesophageal reflux disease (GERD), peptic ulcer disease, and Zollinger–Ellison syndrome. It is also used to prevent up ...
.
Mechanism of action
Saquinavir is a protease inhibitor.Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalysis, catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products ...
s are enzymes that cleave protein molecules into smaller fragments. HIV protease is vital for both viral replication within the cell and release of mature viral particles from an infected cell. Saquinavir binds to the active site of the viral protease and prevents cleavage of viral polyproteins, preventing maturation of the virus. Saquinavir inhibits both HIV-1
The subtypes of HIV include two main subtypes, known as HIV type 1 (HIV-1) and HIV type 2 (HIV-2). These subtypes have distinct genetic differences and are associated with different epidemiological patterns and clinical characteristics.
HIV-1 e ...
and HIV-2 proteases.
History
 Saquinavir was developed by the pharmaceutical company
Saquinavir was developed by the pharmaceutical company Roche
F. Hoffmann-La Roche AG, commonly known as Roche (), is a Switzerland, Swiss multinational corporation, multinational holding healthcare company that operates worldwide under two divisions: Pharmaceuticals and Diagnostics. Its holding company, ...
. Saquinavir was the sixth antiretroviral and the first protease inhibitor approved by the US Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ...
(FDA), leading ritonavir
Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Ritonavir is a protease inhi ...
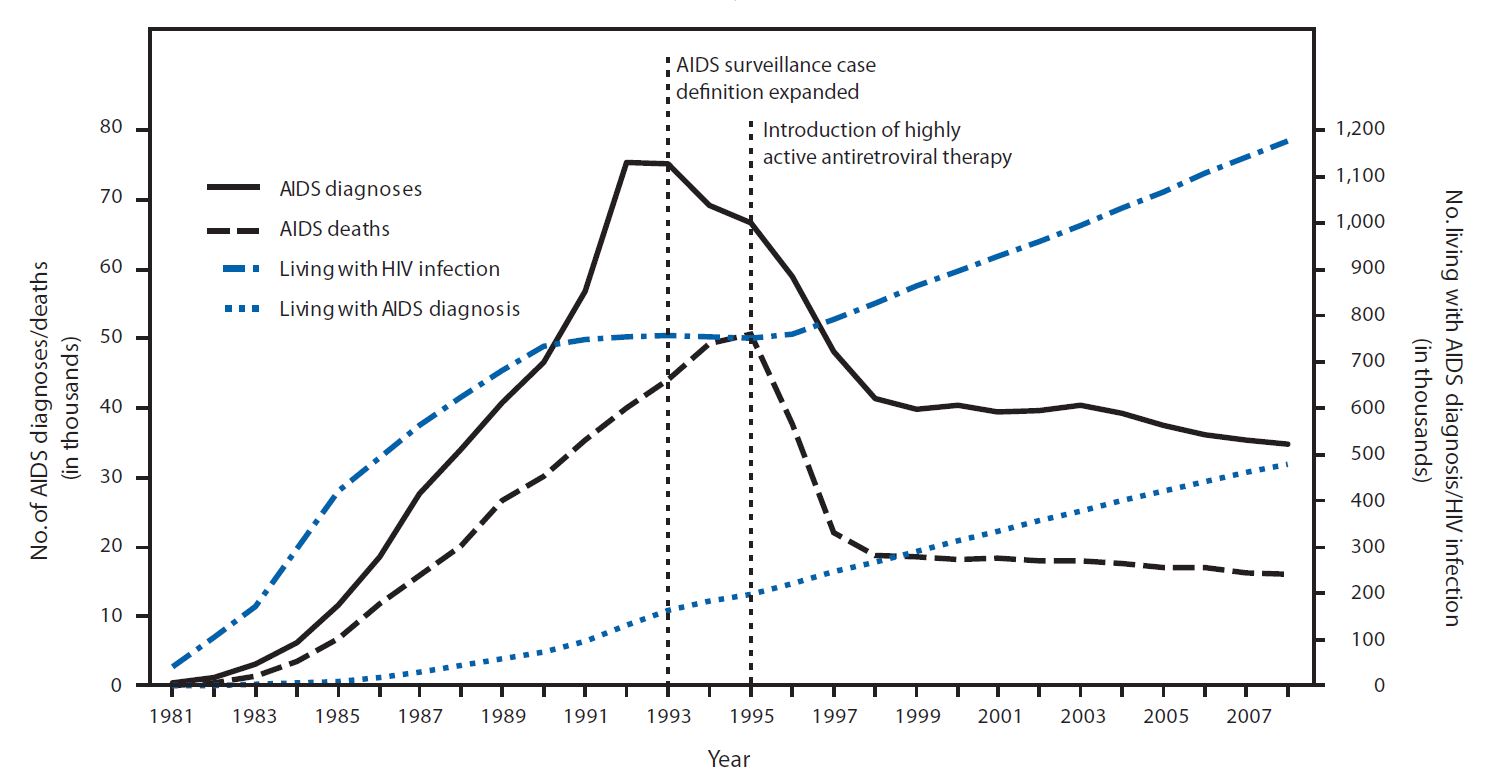
and indinavir by a few months. This new class of antiretrovirals played a critical role in the development of highly active antiretroviral therapy (HAART), which helped significantly lower the risk of death from AIDS-related causes, as seen by a reduction of the annual U.S. HIV-associated death rate, from over 50,000 to about 18,000 over a period of two years.
Roche requested and received approval of Invirase via the FDA's "Accelerated Approval" program—a process designed to speed drugs to market for the treatment of serious diseases—a decision that was controversial, as AIDS activists disagreed over the benefits of thorough testing versus early access to new drugs. It was approved again on November 7, 1997, as Fortovase, a soft gel capsule reformulated for improved bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. H ...
. Roche announced in May 2005 that, given reduced demand, Fortovase would cease being marketed early in 2006, in favor of Invirase boosted with ritonavir
Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Ritonavir is a protease inhi ...
, owing to the ability of the latter co-formulated drug to inhibit the enzyme that metabolizes the AIDS drugs.
Society and culture
Economics
, it is not available as ageneric medication
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active ch ...
.
Formulations
Two formulations have been marketed: * a hard-gel capsule formulation of themesylate
In organosulfur chemistry, a mesylate is any salt or ester of methanesulfonic acid (). In salts, the mesylate is present as the anion. When modifying the international nonproprietary name of a pharmaceutical substance containing the gr ...
, with trade name Invirase, which requires combination with ritonavir
Ritonavir, sold under the brand name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS. This combination treatment is known as highly active antiretroviral therapy (HAART). Ritonavir is a protease inhi ...
to increase the saquinavir bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. H ...
;
* a soft-gel capsule formulation of saquinavir ( microemulsion, orally-administered formulation), with trade name Fortovase, which was discontinued worldwide in 2006.
References
External links
* {{Portal bar , Medicine , Viruses Carboxamides CYP3A4 inhibitors Drugs developed by Hoffmann-La Roche Drugs developed by Genentech Hepatotoxins HIV protease inhibitors Decahydroisoquinolines Quinolines Wikipedia medicine articles ready to translate