Radiation Poisoning (other) on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 Radiation with sufficiently high energy can
Radiation with sufficiently high energy can  Ionizing radiation has many practical uses in medicine, research, and construction, but presents a health hazard if used improperly. Exposure to radiation causes damage to living tissue; high doses result in
Ionizing radiation has many practical uses in medicine, research, and construction, but presents a health hazard if used improperly. Exposure to radiation causes damage to living tissue; high doses result in
 Gamma (γ) radiation consists of photons with a wavelength less than (greater than 1019 Hz and 41.4 keV). Gamma radiation emission is a nuclear process that occurs to rid an unstable
Gamma (γ) radiation consists of photons with a wavelength less than (greater than 1019 Hz and 41.4 keV). Gamma radiation emission is a nuclear process that occurs to rid an unstable
 Alpha particles are
Alpha particles are
 Beta-minus (β−) radiation consists of an energetic electron. It is more penetrating than alpha radiation but less than gamma. Beta radiation from
Beta-minus (β−) radiation consists of an energetic electron. It is more penetrating than alpha radiation but less than gamma. Beta radiation from
 High-energy neutrons are very penetrating and can travel great distances in air (hundreds or even thousands of metres) and moderate distances (several metres) in common solids. They typically require hydrogen rich shielding, such as concrete or water, to block them within distances of less than 1 m. A common source of neutron radiation occurs inside a
High-energy neutrons are very penetrating and can travel great distances in air (hundreds or even thousands of metres) and moderate distances (several metres) in common solids. They typically require hydrogen rich shielding, such as concrete or water, to block them within distances of less than 1 m. A common source of neutron radiation occurs inside a
 The kinetic energy of particles of non-ionizing radiation is too small to produce charged ions when passing through matter. For non-ionizing electromagnetic radiation (see types below), the associated particles (photons) have only sufficient energy to change the rotational, vibrational or electronic valence configurations of molecules and atoms. The effect of non-ionizing forms of radiation on living tissue has only recently been studied. Nevertheless, different biological effects are observed for different types of non-ionizing radiation.
Even "non-ionizing" radiation is capable of causing thermal-ionization if it deposits enough heat to raise temperatures to ionization energies. These reactions occur at far higher total energies than with ionization radiation, which requires only single particles to cause ionization. A familiar example of thermal ionization is the flame-ionization of a common fire, and the browning reactions in common food items induced by infrared radiation, during broiling-type cooking.
The
The kinetic energy of particles of non-ionizing radiation is too small to produce charged ions when passing through matter. For non-ionizing electromagnetic radiation (see types below), the associated particles (photons) have only sufficient energy to change the rotational, vibrational or electronic valence configurations of molecules and atoms. The effect of non-ionizing forms of radiation on living tissue has only recently been studied. Nevertheless, different biological effects are observed for different types of non-ionizing radiation.
Even "non-ionizing" radiation is capable of causing thermal-ionization if it deposits enough heat to raise temperatures to ionization energies. These reactions occur at far higher total energies than with ionization radiation, which requires only single particles to cause ionization. A familiar example of thermal ionization is the flame-ionization of a common fire, and the browning reactions in common food items induced by infrared radiation, during broiling-type cooking.
The
 Radio waves are a type of electromagnetic radiation with wavelengths in the electromagnetic spectrum longer than infrared light. Like all other electromagnetic waves, they travel at the speed of light. Naturally occurring radio waves are made by lightning, or by certain astronomical objects. Artificially generated radio waves are used for fixed and mobile radio communication, broadcasting, radar and other navigation systems, satellite communication, computer networks and innumerable other applications. In addition, almost any wire carrying alternating current will radiate some of the energy away as radio waves; these are mostly termed interference. Different frequencies of radio waves have different propagation characteristics in the Earth's atmosphere; long waves may bend at the rate of the curvature of the Earth and may cover a part of the Earth very consistently, shorter waves travel around the world by multiple reflections off the ionosphere and the Earth. Much shorter wavelengths bend or reflect very little and travel along the line of sight.
Radio waves are a type of electromagnetic radiation with wavelengths in the electromagnetic spectrum longer than infrared light. Like all other electromagnetic waves, they travel at the speed of light. Naturally occurring radio waves are made by lightning, or by certain astronomical objects. Artificially generated radio waves are used for fixed and mobile radio communication, broadcasting, radar and other navigation systems, satellite communication, computer networks and innumerable other applications. In addition, almost any wire carrying alternating current will radiate some of the energy away as radio waves; these are mostly termed interference. Different frequencies of radio waves have different propagation characteristics in the Earth's atmosphere; long waves may bend at the rate of the curvature of the Earth and may cover a part of the Earth very consistently, shorter waves travel around the world by multiple reflections off the ionosphere and the Earth. Much shorter wavelengths bend or reflect very little and travel along the line of sight.
Federal Guidance Report 13
page 16: "For example, the ingestion coefficient risk for 40K would not be appropriate for an application to ingestion of 40K in conjunction with an elevated intake of natural potassium. This is because the biokinetic model for potassium used in this document represents the relatively slow removal of potassium (biological half-time 30 days) that is estimated to occur for typical intakes of potassium, whereas an elevated intake of potassium would result in excretion of a nearly equal mass of natural potassium, and hence of 40K, over a short period." Radiation is ubiquitous on Earth, and humans are adapted to survive at the normal low-to-moderate levels of radiation found on Earth's surface. The relationship between dose and toxicity is often non-linear, and many substances that are toxic at very high doses actually have neutral or positive health effects, or are biologically essential, at moderate or low doses. There is some evidence to suggest that this is true for ionizing radiation: normal levels of ionizing radiation may serve to stimulate and regulate the activity of DNA repair mechanisms. High enough levels of any kind of radiation will eventually become lethal, however.Nancy Trautmann: The Dose Makes the Poison--Or Does It?
Bioscience 2005, American Institute of Biological Sciences Ionizing radiation in certain conditions can damage living organisms, causing cancer or genetic damage. Non-ionizing radiation in certain conditions also can cause damage to living organisms, such as
 On Earth there are different sources of radiation, natural as well as artificial. Natural radiation can come from the Sun, Earth itself, or from
On Earth there are different sources of radiation, natural as well as artificial. Natural radiation can come from the Sun, Earth itself, or from
Health Physics Society Public Education Website
from World Health Organization * ttps://www.bbc.com/news/health-12722435 Q&A: Health effects of radiation exposure ''BBC News'', 21 July 2011. * {{Authority control Physical phenomena
physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge whi ...
, radiation is the emission or transmission of energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
in the form of wave
In physics, mathematics, engineering, and related fields, a wave is a propagating dynamic disturbance (change from List of types of equilibrium, equilibrium) of one or more quantities. ''Periodic waves'' oscillate repeatedly about an equilibrium ...
s or particle
In the physical sciences, a particle (or corpuscle in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from s ...
s through space or a material medium. This includes:
* ''electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
'' consisting of photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s, such as radio wave
Radio waves (formerly called Hertzian waves) are a type of electromagnetic radiation with the lowest frequencies and the longest wavelengths in the electromagnetic spectrum, typically with frequencies below 300 gigahertz (GHz) and wavelengths g ...
s, microwaves
Microwave is a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves. Its wavelength ranges from about one meter to one millimeter, corresponding to frequencies between 300 MHz an ...
, infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
, visible light
Light, visible light, or visible radiation is electromagnetic radiation that can be perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400–700 nanometres (nm ...
, ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
, x-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
s, and gamma radiation (γ)
* ''particle radiation
Particle radiation is the radiation of energy by means of fast-moving subatomic particles. Particle radiation is referred to as a particle beam if the particles are all moving in the same direction, similar to a light beam.
Due to the wave–p ...
'' consisting of particles of non-zero rest energy
The invariant mass, rest mass, intrinsic mass, proper mass, or in the case of bound systems simply mass, is the portion of the total mass of an object or system of objects that is independent of the overall motion of the system. More precisely, ...
, such as alpha radiation
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an atom ...
(α), beta radiation
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus, known as beta decay. There are two forms of beta decay, β− decay and � ...
(β), proton radiation and neutron radiation
Neutron radiation is a form of ionizing radiation that presents as free neutrons. Typical phenomena are nuclear fission or nuclear fusion causing the release of free neutrons, which then react with nuclei of other atoms to form new nuclides— ...
* '' acoustic radiation'', such as ultrasound
Ultrasound is sound with frequency, frequencies greater than 20 Hertz, kilohertz. This frequency is the approximate upper audible hearing range, limit of human hearing in healthy young adults. The physical principles of acoustic waves apply ...
, sound
In physics, sound is a vibration that propagates as an acoustic wave through a transmission medium such as a gas, liquid or solid.
In human physiology and psychology, sound is the ''reception'' of such waves and their ''perception'' by the br ...
, and seismic wave
A seismic wave is a mechanical wave of acoustic energy that travels through the Earth or another planetary body. It can result from an earthquake (or generally, a quake), volcanic eruption, magma movement, a large landslide and a large ma ...
s, all dependent on a physical transmission medium
A transmission medium is a system or substance that can mediate the propagation of signals for the purposes of telecommunication. Signals are typically imposed on a wave of some kind suitable for the chosen medium. For example, data can modula ...
* ''gravitational radiation
Gravitational waves are oscillations of the gravitational field that travel through space at the speed of light; they are generated by the relative motion of gravitating masses. They were proposed by Oliver Heaviside in 1893 and then later by ...
'', in the form of gravitational waves, ripples in spacetime
In physics, spacetime, also called the space-time continuum, is a mathematical model that fuses the three dimensions of space and the one dimension of time into a single four-dimensional continuum. Spacetime diagrams are useful in visualiz ...
Radiation is often categorized as either '' ionizing'' or '' non-ionizing'' depending on the energy of the radiated particles. Ionizing radiation carries more than 10 electron volts (eV), which is enough to ionize
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
atoms and molecules and break chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s. This is an important distinction due to the large difference in harmfulness to living organisms. A common source of ionizing radiation is radioactive materials that emit α, β, or γ radiation, consisting of helium nuclei
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
, electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s or positron
The positron or antielectron is the particle with an electric charge of +1''elementary charge, e'', a Spin (physics), spin of 1/2 (the same as the electron), and the same Electron rest mass, mass as an electron. It is the antiparticle (antimatt ...
s, and photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s, respectively. Other sources include X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
s from medical radiography
Radiography is an imaging technology, imaging technique using X-rays, gamma rays, or similar ionizing radiation and non-ionizing radiation to view the internal form of an object. Applications of radiography include medical ("diagnostic" radiog ...
examinations and muon
A muon ( ; from the Greek letter mu (μ) used to represent it) is an elementary particle similar to the electron, with an electric charge of −1 '' e'' and a spin of ''ħ'', but with a much greater mass. It is classified as a ...
s, meson
In particle physics, a meson () is a type of hadronic subatomic particle composed of an equal number of quarks and antiquarks, usually one of each, bound together by the strong interaction. Because mesons are composed of quark subparticles, the ...
s, positrons, neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s and other particles that constitute the secondary cosmic ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the ...
s that are produced after primary cosmic rays interact with Earth's atmosphere
The atmosphere of Earth is composed of a layer of gas mixture that surrounds the Earth's planetary surface (both lands and oceans), known collectively as air, with variable quantities of suspended aerosols and particulates (which create weathe ...
.
Gamma rays, X-rays, and the higher energy range of ultraviolet light constitute the ionizing part of the electromagnetic spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high ...
. The word "ionize" refers to the breaking of one or more electrons away from an atom, an action that requires the relatively high energies that these electromagnetic waves supply. Further down the spectrum, the non-ionizing lower energies of the lower ultraviolet spectrum cannot ionize atoms, but can disrupt the inter-atomic bonds that form molecules, thereby breaking down molecules rather than atoms; a good example of this is sunburn caused by long-wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
solar ultraviolet. The waves of longer wavelength than UV in visible light, infrared, and microwave frequencies cannot break bonds but can cause vibrations in the bonds which are sensed as heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
. Radio wavelengths and below generally are not regarded as harmful to biological systems. These are not sharp delineations of the energies; there is some overlap in the effects of specific frequencies
Frequency is the number of occurrences of a repeating event per unit of time. Frequency is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena, such as mechanical vibrations, audio ...
.
The word "radiation" arises from the phenomenon of waves ''radiating'' (i.e., traveling outward in all directions) from a source. This aspect leads to a system of measurements and physical units that apply to all types of radiation. Because such radiation expands as it passes through space, and as its energy is conserved (in vacuum), the intensity of all types of radiation from a point source
A point source is a single identifiable ''localized'' source of something. A point source has a negligible extent, distinguishing it from other source geometries. Sources are called point sources because, in mathematical modeling, these sources ...
follows an inverse-square law
In science, an inverse-square law is any scientific law stating that the observed "intensity" of a specified physical quantity is inversely proportional to the square of the distance from the source of that physical quantity. The fundamental ca ...
in relation to the distance from its source. Like any ideal law, the inverse-square law approximates a measured radiation intensity to the extent that the source approximates a geometric point.
Ionizing radiation
 Radiation with sufficiently high energy can
Radiation with sufficiently high energy can ionize
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
atoms; that is to say it can knock electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s off atoms, creating ions. Ionization occurs when an electron is stripped (or "knocked out") from an electron shell of the atom, which leaves the atom with a net positive charge. Because living cells
Cell most often refers to:
* Cell (biology), the functional basic unit of life
* Cellphone, a phone connected to a cellular network
* Clandestine cell, a penetration-resistant form of a secret or outlawed organization
* Electrochemical cell, a d ...
and, more importantly, the DNA in those cells can be damaged by this ionization, exposure to ionizing radiation increases the risk of cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
. Thus "ionizing radiation" is somewhat artificially separated from particle radiation and electromagnetic radiation, simply due to its great potential for biological damage. While an individual cell is made of trillions of atoms, only a small fraction of those will be ionized at low to moderate radiation powers. The probability of ionizing radiation causing cancer is dependent upon the absorbed dose
Absorbed dose is a dose quantity which represents the specific energy (energy per unit mass) deposited by ionizing radiation in living matter. Absorbed dose is used in the calculation of dose uptake in living tissue in both radiation protecti ...
of the radiation and is a function of the damaging tendency of the type of radiation (equivalent dose
Equivalent dose (symbol ''H'') is a dose quantity representing the stochastic health effects of low levels of ionizing radiation on the human body which represents the probability of radiation-induced cancer and genetic damage. It is derived fro ...
) and the sensitivity of the irradiated organism or tissue ( effective dose).
If the source of the ionizing radiation is a radioactive material or a nuclear process such as fission or fusion, there is particle radiation
Particle radiation is the radiation of energy by means of fast-moving subatomic particles. Particle radiation is referred to as a particle beam if the particles are all moving in the same direction, similar to a light beam.
Due to the wave–p ...
to consider. Particle radiation is subatomic particle
In physics, a subatomic particle is a particle smaller than an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles (for example, a baryon, lik ...
s accelerated to relativistic speed
Relativistic speed refers to speed at which relativistic effects become significant to the desired accuracy of measurement of the phenomenon being observed. Relativistic effects are those discrepancies between values calculated by models consider ...
s by nuclear reactions. Because of their momenta
Momenta Global Limited (shortened to Momenta) is a developer of intelligent driving technologies, based in Beijing and Suzhou, China.
History
Momenta was founded in September 2016 led by Cao Xudong, a former scientist at Microsoft Research a ...
, they are quite capable of knocking out electrons and ionizing materials, but since most have an electrical charge, they do not have the penetrating power of ionizing radiation. The exception is neutron particles; see below. There are several different kinds of these particles, but the majority are alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s, beta particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus, known as beta decay. There are two forms of beta decay, β− decay and � ...
s, neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s, and proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s. Roughly speaking, photons and particles with energies above about 10 electron volt
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an electric potential difference of one volt in vacuum. When u ...
s (eV) are ionizing (some authorities use 33 eV, the ionization energy for water). Particle radiation from radioactive material or cosmic rays almost invariably carries enough energy to be ionizing.
Most ionizing radiation originates from radioactive materials and space (cosmic rays), and as such is naturally present in the environment, since most rocks and soil have small concentrations of radioactive materials. Since this radiation is invisible and not directly detectable by human senses, instruments such as Geiger counter
A Geiger counter (, ; also known as a Geiger–Müller counter or G-M counter) is an electronic instrument for detecting and measuring ionizing radiation with the use of a Geiger–Müller tube. It is widely used in applications such as radiat ...
s are usually required to detect its presence. In some cases, it may lead to secondary emission of visible light upon its interaction with matter, as in the case of Cherenkov radiation
Cherenkov radiation () is electromagnetic radiation emitted when a charged particle (such as an electron) passes through a dielectric medium (such as distilled water) at a speed greater than the phase velocity (speed of propagation of a wavefro ...
and radio-luminescence.
 Ionizing radiation has many practical uses in medicine, research, and construction, but presents a health hazard if used improperly. Exposure to radiation causes damage to living tissue; high doses result in
Ionizing radiation has many practical uses in medicine, research, and construction, but presents a health hazard if used improperly. Exposure to radiation causes damage to living tissue; high doses result in Acute radiation syndrome
Acute radiation syndrome (ARS), also known as radiation sickness or radiation poisoning, is a collection of health effects that are caused by being exposed to high amounts of ionizing radiation in a short period of time. Symptoms can start wit ...
(ARS), with skin burns, hair loss, internal organ failure, and death, while any dose may result in an increased chance of cancer and genetic damage
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mitosi ...
; a particular form of cancer, thyroid cancer
Thyroid cancer is cancer that develops from the tissues of the thyroid gland. It is a disease in which cells grow abnormally and have the potential to spread to other parts of the body. Symptoms can include swelling or a lump in the neck, ...
, often occurs when nuclear weapons and reactors are the radiation source because of the biological proclivities of the radioactive iodine fission product, iodine-131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nu ...
. However, calculating the exact risk and chance of cancer forming in cells caused by ionizing radiation is still not well understood, and currently estimates are loosely determined by population-based data from the atomic bombings of Hiroshima and Nagasaki
On 6 and 9 August 1945, the United States detonated two atomic bombs over the Japanese cities of Hiroshima and Nagasaki, respectively, during World War II. The aerial bombings killed between 150,000 and 246,000 people, most of whom were civili ...
and from follow-up of reactor accidents, such as the Chernobyl disaster
On 26 April 1986, the no. 4 reactor of the Chernobyl Nuclear Power Plant, located near Pripyat, Ukrainian Soviet Socialist Republic, Ukrainian SSR, Soviet Union (now Ukraine), exploded. With dozens of direct casualties, it is one of only ...
. The International Commission on Radiological Protection
The International Commission on Radiological Protection (ICRP) is an independent, international, non-governmental organization, with the mission to protect people, animals, and the environment from the harmful effects of ionising radiation. Its ...
states that "The Commission is aware of uncertainties and lack of precision of the models and parameter values", "Collective effective dose is not intended as a tool for epidemiological risk assessment, and it is inappropriate to use it in risk projections" and "in particular, the calculation of the number of cancer deaths based on collective effective doses from trivial individual doses should be avoided".
Ultraviolet radiation
Ultraviolet, of wavelengths from 10 nm to 200 nm, ionizes air molecules, causing it to be strongly absorbed by air and by ozone (O3) in particular. Ionizing UV therefore does not penetrate Earth's atmosphere to a significant degree, and is sometimes referred to asvacuum ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of the ...
. Although present in space, this part of the UV spectrum is not of biological importance, because it does not reach living organisms on Earth.
There is a zone of the atmosphere in which ozone absorbs some 98% of non-ionizing but dangerous UV-C and UV-B. This ozone layer
The ozone layer or ozone shield is a region of Earth's stratosphere that absorption (electromagnetic radiation), absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the a ...
starts at about and extends upward. Some of the ultraviolet spectrum that does reach the ground is non-ionizing, but is still biologically hazardous due to the ability of single photons of this energy to cause electronic excitation in biological molecules, and thus damage them by means of unwanted reactions. An example is the formation of pyrimidine dimer
Pyrimidine dimers represent molecular lesions originating from thymine or cytosine bases within DNA, resulting from photochemical reactions. These lesions, commonly linked to direct DNA damage, are induced by ultraviolet light (UV), particularly ...
s in DNA, which begins at wavelengths below 365 nm (3.4 eV), which is well below ionization energy. This property gives the ultraviolet spectrum some of the dangers of ionizing radiation in biological systems without actual ionization occurring. In contrast, visible light and longer-wavelength electromagnetic radiation, such as infrared, microwaves, and radio waves, consists of photons with too little energy to cause damaging molecular excitation, and thus this radiation is far less hazardous per unit of energy.
X-rays
X-rays are electromagnetic waves with a wavelength less than about 10−9 m (greater than and ). A smaller wavelength corresponds to a higher energy according to the equation ''E'' = ''h'' ''c''/ ''λ''. (''E'' is Energy; ''h'' is the Planck constant; ''c'' is the speed of light; ''λ'' is wavelength.) When an X-ray photon collides with an atom, the atom may absorb the energy of the photon and boost an electron to a higher orbital level, or if the photon is extremely energetic, it may knock an electron from the atom altogether, causing the atom to ionize. Generally, larger atoms are more likely to absorb an X-ray photon since they have greater energy differences between orbital electrons. The soft tissue in the human body is composed of smaller atoms than the calcium atoms that make up bone, so there is a contrast in the absorption of X-rays. X-ray machines are specifically designed to take advantage of the absorption difference between bone and soft tissue, allowing physicians to examine structure in the human body. X-rays are also totally absorbed by the thickness of the earth's atmosphere, resulting in the prevention of the X-ray output of the sun, smaller in quantity than that of UV but nonetheless powerful, from reaching the surface.Gamma radiation
 Gamma (γ) radiation consists of photons with a wavelength less than (greater than 1019 Hz and 41.4 keV). Gamma radiation emission is a nuclear process that occurs to rid an unstable
Gamma (γ) radiation consists of photons with a wavelength less than (greater than 1019 Hz and 41.4 keV). Gamma radiation emission is a nuclear process that occurs to rid an unstable nucleus
Nucleus (: nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucleu ...
of excess energy after most nuclear reactions. Both alpha and beta particles have an electric charge and mass, and thus are quite likely to interact with other atoms in their path. Gamma radiation, however, is composed of photons, which have neither mass nor electric charge and, as a result, penetrates much further through matter than either alpha or beta radiation.
Gamma rays can be stopped by a sufficiently thick or dense layer of material, where the stopping power of the material per given area depends mostly (but not entirely) on the total mass along the path of the radiation, regardless of whether the material is of high or low density. However, as is the case with X-rays, materials with a high atomic number such as lead or depleted uranium
Depleted uranium (DU), also referred to in the past as Q-metal, depletalloy, or D-38, is uranium with a lower content of the fissile isotope Uranium-235, 235U than natural uranium. The less radioactive and non-fissile Uranium-238, 238U is the m ...
add a modest (typically 20% to 30%) amount of stopping power over an equal mass of less dense and lower atomic weight materials (such as water or concrete). The atmosphere absorbs all gamma rays approaching Earth from space. Even air is capable of absorbing gamma rays, halving the energy of such waves by passing through, on the average, .
Alpha radiation
 Alpha particles are
Alpha particles are helium-4
Helium-4 () is a stable isotope of the element helium. It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consi ...
nuclei (two protons and two neutrons). They interact with matter strongly due to their charges and combined mass, and at their usual velocities only penetrate a few centimetres of air, or a few millimetres of low density material (such as the thin mica material which is specially placed in some Geiger counter tubes to allow alpha particles in). This means that alpha particles from ordinary alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
do not penetrate the outer layers of dead skin cells and cause no damage to the live tissues below. Some very high energy alpha particles compose about 10% of cosmic ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the ...
s, and these are capable of penetrating the body and even thin metal plates. However, they are of danger only to astronauts, since they are deflected by the Earth's magnetic field and then stopped by its atmosphere.
Alpha radiation is dangerous when alpha-emitting radioisotopes
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
are inhaled or ingested (breathed or swallowed). This brings the radioisotope close enough to sensitive live tissue for the alpha radiation to damage cells. Per unit of energy, alpha particles are at least 20 times more effective at cell-damage than gamma rays and X-rays. See relative biological effectiveness
In radiobiology, the relative biological effectiveness (often abbreviated as RBE) is the ratio of biological effectiveness of one type of ionizing radiation relative to another, given the same amount of absorbed energy. The RBE is an empirical ...
for a discussion of this. Examples of highly poisonous alpha-emitters are all isotopes of radium
Radium is a chemical element; it has chemical symbol, symbol Ra and atomic number 88. It is the sixth element in alkaline earth metal, group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, ...
, radon
Radon is a chemical element; it has symbol Rn and atomic number 86. It is a radioactive noble gas and is colorless and odorless. Of the three naturally occurring radon isotopes, only Rn has a sufficiently long half-life (3.825 days) for it to b ...
, and polonium
Polonium is a chemical element; it has symbol Po and atomic number 84. A rare and highly radioactive metal (although sometimes classified as a metalloid) with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tel ...
, due to the amount of decay that occur in these short half-life materials.
Beta radiation
 Beta-minus (β−) radiation consists of an energetic electron. It is more penetrating than alpha radiation but less than gamma. Beta radiation from
Beta-minus (β−) radiation consists of an energetic electron. It is more penetrating than alpha radiation but less than gamma. Beta radiation from radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
can be stopped with a few centimetres of plastic or a few millimetres of metal. It occurs when a neutron decays into a proton in a nucleus, releasing the beta particle and an antineutrino
A neutrino ( ; denoted by the Greek letter ) is an elementary particle that interacts via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass is so small ('' -ino'') that it ...
. Beta radiation from linac
A linear particle accelerator (often shortened to linac) is a type of particle accelerator that accelerates charged subatomic particles or ions to a high speed by subjecting them to a series of oscillating electric potentials along a linear bea ...
accelerators is far more energetic and penetrating than natural beta radiation. It is sometimes used therapeutically in radiotherapy
Radiation therapy or radiotherapy (RT, RTx, or XRT) is a treatment using ionizing radiation, generally provided as part of cancer therapy to either kill or control the growth of malignant cells. It is normally delivered by a linear particle ...
to treat superficial tumors.
Beta-plus (β+) radiation is the emission of positron
The positron or antielectron is the particle with an electric charge of +1''elementary charge, e'', a Spin (physics), spin of 1/2 (the same as the electron), and the same Electron rest mass, mass as an electron. It is the antiparticle (antimatt ...
s, which are the antimatter
In modern physics, antimatter is defined as matter composed of the antiparticles (or "partners") of the corresponding subatomic particle, particles in "ordinary" matter, and can be thought of as matter with reversed charge and parity, or go ...
form of electrons. When a positron slows to speeds similar to those of electrons in the material, the positron will annihilate an electron, releasing two gamma photons of 511 keV in the process. Those two gamma photons will be traveling in (approximately) opposite directions. The gamma radiation from positron annihilation consists of high energy photons, and is also ionizing.
Neutron radiation
Neutrons are categorized according to their speed/energy. Neutron radiation consists offree neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The neutron was discovered by James Chadwick in 1932, leading to the discovery of nuclear fission in 1938, the f ...
s. These neutrons may be emitted during either spontaneous or induced nuclear fission. Neutrons are rare radiation particles; they are produced in large numbers only where chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sys ...
fission or fusion reactions are active; this happens for about 10 microseconds in a thermonuclear explosion, or continuously inside an operating nuclear reactor; production of the neutrons stops almost immediately in the reactor when it goes non-critical.
Neutrons can make other objects, or material, radioactive. This process, called neutron activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emi ...
, is the primary method used to produce radioactive sources for use in medical, academic, and industrial applications. Even comparatively low speed thermal neutron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium wit ...
s cause neutron activation (in fact, they cause it more efficiently). Neutrons do not ionize atoms in the same way that charged particles such as protons and electrons do (by the excitation of an electron), because neutrons have no charge. It is through their absorption by nuclei which then become unstable that they cause ionization. Hence, neutrons are said to be "indirectly ionizing". Even neutrons without significant kinetic energy are indirectly ionizing, and are thus a significant radiation hazard. Not all materials are capable of neutron activation; in water, for example, the most common isotopes of both types atoms present (hydrogen and oxygen) capture neutrons and become heavier but remain stable forms of those atoms. Only the absorption of more than one neutron, a statistically rare occurrence, can activate a hydrogen atom, while oxygen requires two additional absorptions. Thus water is only very weakly capable of activation. The sodium in salt (as in sea water), on the other hand, need only absorb a single neutron to become Na-24, a very intense source of beta decay, with a half-life of 15 hours.
In addition, high-energy (high-speed) neutrons have the ability to directly ionize atoms. One mechanism by which high energy neutrons ionize atoms is to strike the nucleus of an atom and knock the atom out of a molecule, leaving one or more electrons behind as the chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
is broken. This leads to production of chemical free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
s. In addition, very high energy neutrons can cause ionizing radiation by "neutron spallation" or knockout, wherein neutrons cause emission of high-energy protons from atomic nuclei (especially hydrogen nuclei) on impact. The last process imparts most of the neutron's energy to the proton, much like one billiard ball
A billiard ball is a small, hard ball used in cue sports, such as carom billiards, pool, and snooker. The number, type, diameter, color, and pattern of the balls differ depending upon the specific game being played. Various particular ball pro ...
striking another. The charged protons and other products from such reactions are directly ionizing.
 High-energy neutrons are very penetrating and can travel great distances in air (hundreds or even thousands of metres) and moderate distances (several metres) in common solids. They typically require hydrogen rich shielding, such as concrete or water, to block them within distances of less than 1 m. A common source of neutron radiation occurs inside a
High-energy neutrons are very penetrating and can travel great distances in air (hundreds or even thousands of metres) and moderate distances (several metres) in common solids. They typically require hydrogen rich shielding, such as concrete or water, to block them within distances of less than 1 m. A common source of neutron radiation occurs inside a nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
, where a metres-thick water layer is used as effective shielding.
Cosmic radiation
There are two sources of high energy particles entering the Earth's atmosphere from outer space: the sun and deep space. The sun continuously emits particles, primarily free protons, in the solar wind, and occasionally augments the flow hugely withcoronal mass ejection
A coronal mass ejection (CME) is a significant ejection of plasma mass from the Sun's corona into the heliosphere. CMEs are often associated with solar flares and other forms of solar activity, but a broadly accepted theoretical understandin ...
s (CME).
The particles from deep space (inter- and extra-galactic) are much less frequent, but of much higher energies. These particles are also mostly protons, with much of the remainder consisting of helions (alpha particles). A few completely ionized nuclei of heavier elements are present. The origin of these galactic cosmic rays is not yet well understood, but they seem to be remnants of supernova
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last stellar evolution, evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion ...
e and especially gamma-ray burst
In gamma-ray astronomy, gamma-ray bursts (GRBs) are extremely energetic events occurring in distant Galaxy, galaxies which represent the brightest and most powerful class of explosion in the universe. These extreme Electromagnetic radiation, ele ...
s (GRB), which feature magnetic fields capable of the huge accelerations measured from these particles. They may also be generated by quasar
A quasar ( ) is an extremely Luminosity, luminous active galactic nucleus (AGN). It is sometimes known as a quasi-stellar object, abbreviated QSO. The emission from an AGN is powered by accretion onto a supermassive black hole with a mass rangi ...
s, which are galaxy-wide jet phenomena similar to GRBs but known for their much larger size, and which seem to be a violent part of the universe's early history.
Non-ionizing radiation
 The kinetic energy of particles of non-ionizing radiation is too small to produce charged ions when passing through matter. For non-ionizing electromagnetic radiation (see types below), the associated particles (photons) have only sufficient energy to change the rotational, vibrational or electronic valence configurations of molecules and atoms. The effect of non-ionizing forms of radiation on living tissue has only recently been studied. Nevertheless, different biological effects are observed for different types of non-ionizing radiation.
Even "non-ionizing" radiation is capable of causing thermal-ionization if it deposits enough heat to raise temperatures to ionization energies. These reactions occur at far higher total energies than with ionization radiation, which requires only single particles to cause ionization. A familiar example of thermal ionization is the flame-ionization of a common fire, and the browning reactions in common food items induced by infrared radiation, during broiling-type cooking.
The
The kinetic energy of particles of non-ionizing radiation is too small to produce charged ions when passing through matter. For non-ionizing electromagnetic radiation (see types below), the associated particles (photons) have only sufficient energy to change the rotational, vibrational or electronic valence configurations of molecules and atoms. The effect of non-ionizing forms of radiation on living tissue has only recently been studied. Nevertheless, different biological effects are observed for different types of non-ionizing radiation.
Even "non-ionizing" radiation is capable of causing thermal-ionization if it deposits enough heat to raise temperatures to ionization energies. These reactions occur at far higher total energies than with ionization radiation, which requires only single particles to cause ionization. A familiar example of thermal ionization is the flame-ionization of a common fire, and the browning reactions in common food items induced by infrared radiation, during broiling-type cooking.
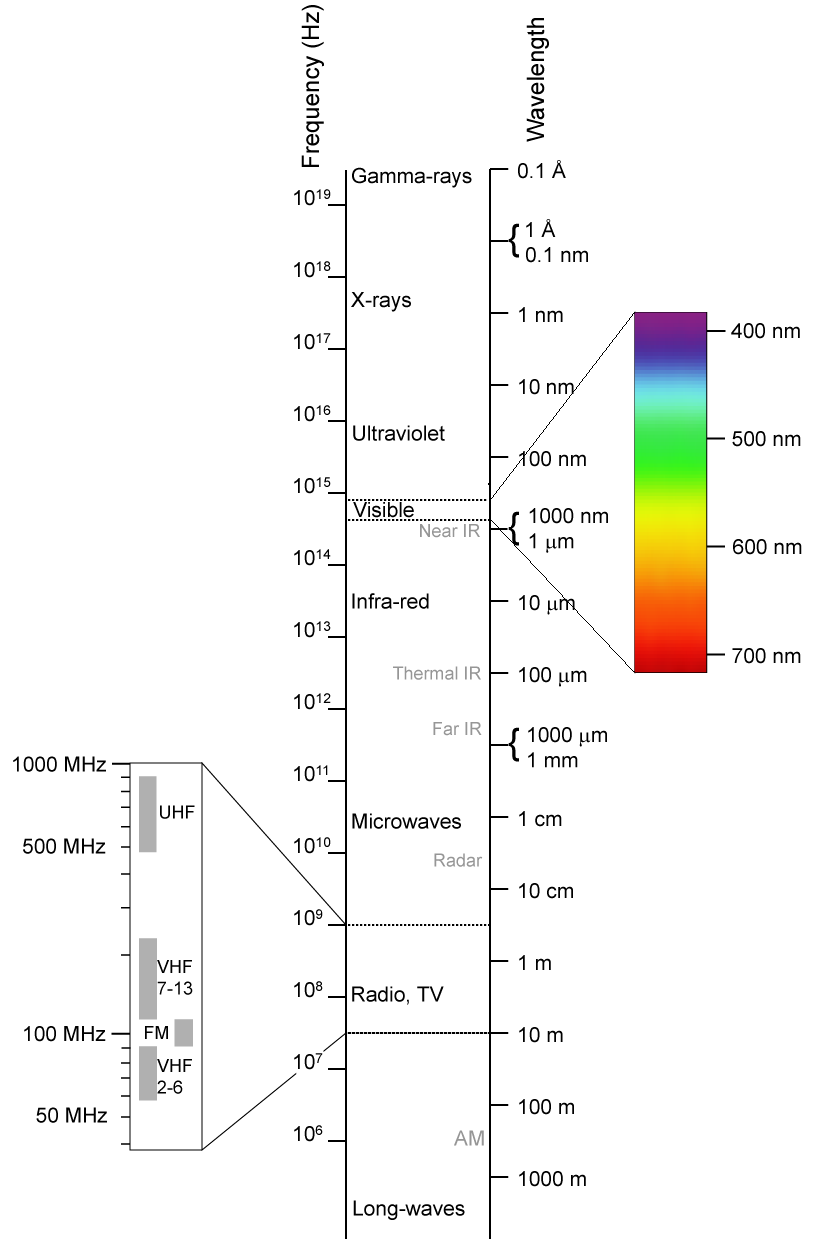
The electromagnetic spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high ...
is the range of all possible electromagnetic radiation frequencies. The electromagnetic spectrum (usually just spectrum) of an object is the characteristic distribution of electromagnetic radiation emitted by, or absorbed by, that particular object.
The non-ionizing portion of electromagnetic radiation consists of electromagnetic waves that (as individual quanta or particles, see photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
) are not energetic enough to detach electrons from atoms or molecules and hence cause their ionization. These include radio waves, microwaves, infrared, and (sometimes) visible light. The lower frequencies of ultraviolet light may cause chemical changes and molecular damage similar to ionization, but is technically not ionizing. The highest frequencies of ultraviolet light, as well as all X-rays and gamma-rays are ionizing.
The occurrence of ionization depends on the energy of the individual particles or waves, and not on their number. An intense flood of particles or waves will not cause ionization if these particles or waves do not carry enough energy to be ionizing, unless they raise the temperature of a body to a point high enough to ionize small fractions of atoms or molecules by the process of thermal-ionization (this, however, requires relatively extreme radiation intensities).
Ultraviolet light
As noted above, the lower part of the spectrum of ultraviolet, called soft UV, from 3 eV to about 10 eV, is non-ionizing. However, the effects of non-ionizing ultraviolet on chemistry and the damage to biological systems exposed to it (including oxidation, mutation, and cancer) are such that even this part of ultraviolet is often compared with ionizing radiation.Visible light
Light, or visible light, is a very narrow range of electromagnetic radiation of a wavelength that is visible to the human eye, or 380–750 nm which equates to a frequency range of 790 to 400 THz respectively. More broadly, physicists use the term "light" to mean electromagnetic radiation of all wavelengths, whether visible or not.Infrared
Infrared (IR) light is electromagnetic radiation with a wavelength between 0.7 and 300 μm, which corresponds to a frequency range between 430 and 1 THz respectively. IR wavelengths are longer than that of visible light, but shorter than that of microwaves. Infrared may be detected at a distance from the radiating objects by "feel". Infrared sensing snakes can detect and focus infrared by use of a pinhole lens in their heads, called "pits". Bright sunlight provides an irradiance of just over 1 kW/m2 at sea level. Of this energy, 53% is infrared radiation, 44% is visible light, and 3% is ultraviolet radiation.Microwave
Microwaves are electromagnetic waves with wavelengths ranging from as short as 1 mm to as long as 1 m, which equates to a frequency range of 300 MHz to 300 GHz. This broad definition includes both UHF and EHF (millimetre waves), but various sources use different other limits. In all cases, microwaves include the entire super high frequency band (3 to 30 GHz, or 10 to 1 cm) at minimum, with RF engineering often putting the lower boundary at 1 GHz (30 cm), and the upper around 100 GHz (3 mm).Radio waves
Very low frequency
Very low frequency (VLF) refers to a frequency range of 30 Hz to 3 kHz which corresponds to wavelengths of respectively. Since there is not much bandwidth in this range of the radio spectrum, only the very simplest signals can be transmitted, such as for radio navigation. Also known as themyriametre
The following are examples of order of magnitude, orders of magnitude for different lengths.
Overview
Detailed list
To help compare different orders of magnitude, the following list describes various lengths between 1.6 \times 10^ me ...
band or myriametre wave as the wavelengths range from 100 km to 10 km (an obsolete metric unit equal to 10 km).
Extremely low frequency
Extremely low frequency (ELF) is radiation frequencies from 3 to 30 Hz (108 to 107 m respectively). In atmosphere science, an alternative definition is usually given, from 3 Hz to 3 kHz. In the related magnetosphere science, the lower frequency electromagnetic oscillations (pulsations occurring below ~3 Hz) are considered to lie in the ULF range, which is thus also defined differently from the ITU Radio Bands. A massive military ELF antenna in Michigan radiates very slow messages to otherwise unreachable receivers, such as submerged submarines.Thermal radiation (heat)
Thermal radiation is a common synonym for infrared radiation emitted by objects at temperatures often encountered on Earth. Thermal radiation refers not only to the radiation itself, but also the process by which the surface of an object radiates itsthermal energy
The term "thermal energy" is often used ambiguously in physics and engineering. It can denote several different physical concepts, including:
* Internal energy: The energy contained within a body of matter or radiation, excluding the potential en ...
in the form of black-body radiation. Infrared or red radiation from a common household radiator or electric heater is an example of thermal radiation, as is the heat emitted by an operating incandescent light bulb. Thermal radiation is generated when energy from the movement of charged particles within atoms is converted to electromagnetic radiation.
As noted above, even low-frequency thermal radiation may cause temperature-ionization whenever it deposits sufficient thermal energy to raise temperatures to a high enough level. Common examples of this are the ionization (plasma) seen in common flames, and the molecular changes caused by the " browning" during food-cooking, which is a chemical process that begins with a large component of ionization.
Black-body radiation
''Black-body
A black body or blackbody is an idealized physical body that absorbs all incident electromagnetic radiation, regardless of frequency or angle of incidence. The radiation emitted by a black body in thermal equilibrium with its environment is ...
radiation'' is an idealized spectrum of radiation emitted by a body that is at a uniform temperature. The shape of the spectrum and the total amount of energy emitted by the body is a function of the absolute temperature of that body. The radiation emitted covers the entire electromagnetic spectrum and the intensity of the radiation (power/unit-area) at a given frequency is described by Planck's law
In physics, Planck's law (also Planck radiation law) describes the spectral density of electromagnetic radiation emitted by a black body in thermal equilibrium at a given temperature , when there is no net flow of matter or energy between the ...
of radiation. For a given temperature of a black-body there is a particular frequency at which the radiation emitted is at its maximum intensity. That maximum radiation frequency moves toward higher frequencies as the temperature of the body increases. The frequency at which the black-body radiation is at maximum is given by Wien's displacement law
In physics, Wien's displacement law states that the black-body radiation curve for different temperatures will peak at different wavelengths that are inversely proportional to the temperature. The shift of that peak is a direct consequence of ...
and is a function of the body's absolute temperature. A black-body is one that emits at any temperature the maximum possible amount of radiation at any given wavelength. A black-body will also absorb the maximum possible incident radiation at any given wavelength. A black-body with a temperature at or below room temperature would thus appear absolutely black, as it would not reflect any incident light nor would it emit enough radiation at visible wavelengths for our eyes to detect. Theoretically, a black-body emits electromagnetic radiation over the entire spectrum from very low frequency radio waves to x-rays, creating a continuum of radiation.
The color of a radiating black-body tells the temperature of its radiating surface. It is responsible for the color of stars
A star is a luminous spheroid of plasma held together by self-gravity. The nearest star to Earth is the Sun. Many other stars are visible to the naked eye at night; their immense distances from Earth make them appear as fixed points of ...
, which vary from infrared through red (), to yellow (), to white and to blue-white () as the peak radiance passes through those points in the visible spectrum. When the peak is below the visible spectrum the body is black, while when it is above the body is blue-white, since all the visible colors are represented from blue decreasing to red.
Discovery
Electromagnetic radiation of wavelengths other than visible light were discovered in the early 19th century. The discovery of infrared radiation is ascribed toWilliam Herschel
Frederick William Herschel ( ; ; 15 November 1738 – 25 August 1822) was a German-British astronomer and composer. He frequently collaborated with his younger sister and fellow astronomer Caroline Herschel. Born in the Electorate of Hanover ...
, the astronomer
An astronomer is a scientist in the field of astronomy who focuses on a specific question or field outside the scope of Earth. Astronomers observe astronomical objects, such as stars, planets, natural satellite, moons, comets and galaxy, galax ...
. Herschel published his results in 1800 before the Royal Society of London
The Royal Society, formally The Royal Society of London for Improving Natural Knowledge, is a learned society and the United Kingdom's national academy of sciences. The society fulfils a number of roles: promoting science and its benefits, r ...
. Herschel, like Ritter, used a prism
PRISM is a code name for a program under which the United States National Security Agency (NSA) collects internet communications from various U.S. internet companies. The program is also known by the SIGAD . PRISM collects stored internet ...
to refract
In physics, refraction is the redirection of a wave as it passes from one medium to another. The redirection can be caused by the wave's change in speed or by a change in the medium. Refraction of light is the most commonly observed phenome ...
light from the Sun
The Sun is the star at the centre of the Solar System. It is a massive, nearly perfect sphere of hot plasma, heated to incandescence by nuclear fusion reactions in its core, radiating the energy from its surface mainly as visible light a ...
and detected the infrared (beyond the red
Red is the color at the long wavelength end of the visible spectrum of light, next to orange and opposite violet. It has a dominant wavelength of approximately 625–750 nanometres. It is a primary color in the RGB color model and a seconda ...
part of the spectrum), through an increase in the temperature recorded by a thermometer
A thermometer is a device that measures temperature (the hotness or coldness of an object) or temperature gradient (the rates of change of temperature in space). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb ...
.
In 1801, the German physicist Johann Wilhelm Ritter
Johann Wilhelm Ritter (16 December 1776 – 23 January 1810). was a German chemist, physicist and philosopher. He was born in Samitz (Zamienice) near Haynau (Chojnów) in Silesia (then part of Prussia, since 1945 in Poland), and died in Muni ...
made the discovery of ultraviolet by noting that the rays from a prism darkened silver chloride
Silver chloride is an inorganic chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water and its sensitivity to light. Upon illumination or heating, silver chloride converts ...
preparations more quickly than violet light. Ritter's experiments were an early precursor to what would become photography. Ritter noted that the UV rays were capable of causing chemical reactions.
The first radio waves detected were not from a natural source, but were produced deliberately and artificially by the German scientist Heinrich Hertz
Heinrich Rudolf Hertz (; ; 22 February 1857 – 1 January 1894) was a German physicist who first conclusively proved the existence of the electromagnetic waves predicted by James Clerk Maxwell's equations of electromagnetism.
Biography
Heinri ...
in 1887, using electrical circuits calculated to produce oscillations in the radio frequency range, following formulas suggested by the equations of James Clerk Maxwell
James Clerk Maxwell (13 June 1831 – 5 November 1879) was a Scottish physicist and mathematician who was responsible for the classical theory of electromagnetic radiation, which was the first theory to describe electricity, magnetism an ...
.
Wilhelm Röntgen
Wilhelm Conrad Röntgen (; 27 March 1845 – 10 February 1923), sometimes Transliteration, transliterated as Roentgen ( ), was a German physicist who produced and detected electromagnetic radiation in a wavelength range known as X-rays. As ...
discovered and named X-rays
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
. While experimenting with high voltages applied to an evacuated tube on 8 November 1895, he noticed a fluorescence on a nearby plate of coated glass. Within a month, he discovered the main properties of X-rays that we understand to this day.
In 1896, Henri Becquerel
Antoine Henri Becquerel ( ; ; 15 December 1852 – 25 August 1908) was a French nuclear physicist who shared the 1903 Nobel Prize in Physics with Marie and Pierre Curie for his discovery of radioactivity.
Biography
Family and education
Becq ...
found that rays emanating from certain minerals penetrated black paper and caused fogging of an unexposed photographic plate. His doctoral student Marie Curie
Maria Salomea Skłodowska-Curie (; ; 7 November 1867 – 4 July 1934), known simply as Marie Curie ( ; ), was a Polish and naturalised-French physicist and chemist who conducted pioneering research on radioactivity.
She was List of female ...
discovered that only certain chemical elements gave off these rays of energy. She named this behavior radioactivity
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
.
Alpha rays (alpha particles) and beta rays (beta particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus, known as beta decay. There are two forms of beta decay, β− decay and � ...
s) were differentiated by Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson (30 August 1871 – 19 October 1937) was a New Zealand physicist who was a pioneering researcher in both Atomic physics, atomic and nuclear physics. He has been described as "the father of nu ...
through simple experimentation in 1899. Rutherford used a generic pitchblende radioactive source and determined that the rays produced by the source had differing penetrations in materials. One type had short penetration (it was stopped by paper) and a positive charge, which Rutherford named ''alpha rays''. The other was more penetrating (able to expose film through paper but not metal) and had a negative charge, and this type Rutherford named ''beta''. This was the radiation that had been first detected by Becquerel from uranium salts. In 1900, the French scientist Paul Villard
Paul Ulrich Villard (28 September 1860 – 13 January 1934) was a French chemist and physicist. He discovered gamma rays in 1900 while studying the radiation emanating from radium.
Early research
Villard was born in Saint-Germain-au-Mo ...
discovered a third neutrally charged and especially penetrating type of radiation from radium, and after he described it, Rutherford realized it must be yet a third type of radiation, which in 1903 Rutherford named gamma ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
s.
Henri Becquerel himself proved that beta rays are fast electrons, while Rutherford and Thomas Royds proved in 1909 that alpha particles are ionized helium. Rutherford and Edward Andrade
Edward Neville da Costa Andrade FRS ( 27 December 1887 – 6 June 1971) was an English physicist, writer, and poet. He told ''The Literary Digest'' his name was pronounced "as written, i.e., like ''air raid'', with ''and'' substituted for ''a ...
proved in 1914 that gamma rays are like X-rays, but with shorter wavelengths.
Cosmic ray radiations striking the Earth from outer space were finally definitively recognized and proven to exist in 1912, as the scientist Victor Hess
Victor Franz Hess (; 24 June 1883 – 17 December 1964) was an Austrian-American particle physicist who shared the 1936 Nobel Prize in Physics with Carl David Anderson "for his discovery of cosmic radiation".
Biography
He was born to Vinzenz H ...
carried an electrometer
An electrometer is an electrical instrument for measuring electric charge or electrical potential difference. There are many different types, ranging from historical handmade mechanical instruments to high-precision electronic devices. Modern ...
to various altitudes in a free balloon flight. The nature of these radiations was only gradually understood in later years.
The neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
and neutron radiation
Neutron radiation is a form of ionizing radiation that presents as free neutrons. Typical phenomena are nuclear fission or nuclear fusion causing the release of free neutrons, which then react with nuclei of other atoms to form new nuclides— ...
were discovered by James Chadwick
Sir James Chadwick (20 October 1891 – 24 July 1974) was an English nuclear physicist who received the Nobel Prize in Physics in 1935 for his discovery of the neutron. In 1941, he wrote the final draft of the MAUD Report, which inspired t ...
in 1932. A number of other high energy particulate radiations such as positron
The positron or antielectron is the particle with an electric charge of +1''elementary charge, e'', a Spin (physics), spin of 1/2 (the same as the electron), and the same Electron rest mass, mass as an electron. It is the antiparticle (antimatt ...
s, muon
A muon ( ; from the Greek letter mu (μ) used to represent it) is an elementary particle similar to the electron, with an electric charge of −1 '' e'' and a spin of ''ħ'', but with a much greater mass. It is classified as a ...
s, and pion
In particle physics, a pion (, ) or pi meson, denoted with the Greek alphabet, Greek letter pi (letter), pi (), is any of three subatomic particles: , , and . Each pion consists of a quark and an antiquark and is therefore a meson. Pions are the ...
s were discovered by cloud chamber examination of cosmic ray reactions shortly thereafter, and others types of particle radiation were produced artificially in particle accelerators
A particle accelerator is a machine that uses electromagnetic fields to propel electric charge, charged particles to very high speeds and energies to contain them in well-defined particle beam, beams. Small accelerators are used for fundamental ...
, through the last half of the twentieth century.
Applications
Medicine
Radiation and radioactive substances are used for diagnosis, treatment, and research. X-rays, for example, pass through muscles and other soft tissue but are stopped by dense materials. This property of X-rays enables doctors to find broken bones and to locate cancers that might be growing in the body. Doctors also find certain diseases by injecting a radioactive substance and monitoring the radiation given off as the substance moves through the body. Radiation used for cancer treatment is called ionizing radiation because it forms ions in the cells of the tissues it passes through as it dislodges electrons from atoms. This can kill cells or change genes so the cells cannot grow. Other forms of radiation such as radio waves, microwaves, and light waves are called non-ionizing. They do not have as much energy so they are not able to ionize cells.Communication
All modern communication systems use forms of electromagnetic radiation. Variations in the intensity of the radiation represent changes in the sound, pictures, or other information being transmitted. For example, a human voice can be sent as a radio wave or microwave by making the wave vary to corresponding variations in the voice. Musicians have also experimented with gamma rays sonification, or using nuclear radiation, to produce sound and music.Science
Researchers use radioactive atoms to determine the age of materials that were once part of a living organism. The age of such materials can be estimated by measuring the amount of radioactive carbon they contain in a process calledradiocarbon dating
Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) is a method for Chronological dating, determining the age of an object containing organic material by using the properties of carbon-14, radiocarbon, a radioactive Isotop ...
. Similarly, using other radioactive elements, the age of rocks and other geological features (even some man-made objects) can be determined; this is called Radiometric dating
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to Chronological dating, date materials such as Rock (geology), rocks or carbon, in which trace radioactive impurity, impurities were selectively incorporat ...
. Environmental scientists use radioactive atoms, known as tracer atoms, to identify the pathways taken by pollutants through the environment.
Radiation is used to determine the composition of materials in a process called neutron activation analysis
Neutron activation analysis (NAA) is a nuclear reaction, nuclear process used for determining the concentrations of chemical element, elements in many materials. NAA allows discrete Sampling (statistics), sampling of elements as it disregards the ...
. In this process, scientists bombard a sample of a substance with particles called neutrons. Some of the atoms in the sample absorb neutrons and become radioactive. The scientists can identify the elements in the sample by studying the emitted radiation.
Possible damage to health and environment from certain types of radiation
Radiation is not always dangerous, and not all types of radiation are equally dangerous, contrary to several common medical myths. For example, althoughbananas
A banana is an elongated, edible fruit – berry (botany), botanically a berry – produced by several kinds of large treelike herbaceous flowering plants in the genus ''Musa (genus), Musa''. In some countries, cooking bananas are called pla ...
contain naturally occurring radioactive isotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
s, particularly potassium-40
Potassium-40 (K) is a long lived and the main naturally occurring radioactive isotope of potassium. Its half-life is 1.25 billion years. It makes up about 0.012% (120 parts-per notation, ppm) of natural potassium.
Potassium-40 undergoes four dif ...
(40K), which emit ionizing radiation when undergoing radioactive decay, the levels of such radiation are far too low to induce radiation poisoning
Acute radiation syndrome (ARS), also known as radiation sickness or radiation poisoning, is a collection of health effects that are caused by being exposed to high amounts of ionizing radiation in a short period of time. Symptoms can start wit ...
, and bananas are not a radiation hazard. It would not be physically possible to eat enough bananas to cause radiation poisoning, as the radiation dose from bananas is non-cumulative.U. S. Environmental Protection Agency (1999)Federal Guidance Report 13
page 16: "For example, the ingestion coefficient risk for 40K would not be appropriate for an application to ingestion of 40K in conjunction with an elevated intake of natural potassium. This is because the biokinetic model for potassium used in this document represents the relatively slow removal of potassium (biological half-time 30 days) that is estimated to occur for typical intakes of potassium, whereas an elevated intake of potassium would result in excretion of a nearly equal mass of natural potassium, and hence of 40K, over a short period." Radiation is ubiquitous on Earth, and humans are adapted to survive at the normal low-to-moderate levels of radiation found on Earth's surface. The relationship between dose and toxicity is often non-linear, and many substances that are toxic at very high doses actually have neutral or positive health effects, or are biologically essential, at moderate or low doses. There is some evidence to suggest that this is true for ionizing radiation: normal levels of ionizing radiation may serve to stimulate and regulate the activity of DNA repair mechanisms. High enough levels of any kind of radiation will eventually become lethal, however.Nancy Trautmann: The Dose Makes the Poison--Or Does It?
Bioscience 2005, American Institute of Biological Sciences Ionizing radiation in certain conditions can damage living organisms, causing cancer or genetic damage. Non-ionizing radiation in certain conditions also can cause damage to living organisms, such as
burn
A burn is an injury to skin, or other tissues, caused by heat, electricity, chemicals, friction, or ionizing radiation (such as sunburn, caused by ultraviolet radiation). Most burns are due to heat from hot fluids (called scalding), soli ...
s. In 2011, the International Agency for Research on Cancer
The International Agency for Research on Cancer (IARC; ) is an intergovernmental agency forming part of the World Health Organization of the United Nations.
Its role is to conduct and coordinate research into the causes of cancer. It also cance ...
(IARC) of the World Health Organization
The World Health Organization (WHO) is a list of specialized agencies of the United Nations, specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Gen ...
(WHO) released a statement adding radio frequency electromagnetic fields (including microwave and millimetre waves) to their list of things which are possibly carcinogenic to humans.
RWTH Aachen University's EMF-Portal web site presents one of the biggest database about the effects of electromagnetic radiation
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength ...
. As of 12 July 2019 it has 28,547 publications and 6,369 summaries of individual scientific studies on the effects of electromagnetic fields.
Environmental radioactivity
 On Earth there are different sources of radiation, natural as well as artificial. Natural radiation can come from the Sun, Earth itself, or from
On Earth there are different sources of radiation, natural as well as artificial. Natural radiation can come from the Sun, Earth itself, or from cosmic radiation
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Sol ...
.
See also
* Australian Radiation Protection and Nuclear Safety Agency (ARPANSA) * Background radiation, which actually refers to background ionizing radiation *Cherenkov radiation
Cherenkov radiation () is electromagnetic radiation emitted when a charged particle (such as an electron) passes through a dielectric medium (such as distilled water) at a speed greater than the phase velocity (speed of propagation of a wavefro ...
* Cosmic microwave background radiation
The cosmic microwave background (CMB, CMBR), or relic radiation, is microwave radiation that fills all space in the observable universe. With a standard optical telescope, the background space between stars and galaxies is almost completely dar ...
, 3 K blackbody radiation that fills the Universe
* Electromagnetic spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high ...
* FASTRAD
FASTRAD is a tool dedicated to the calculation of radiation effects (Dose and Displacement Damage) on electronics. The software has uses in high energy physics and nuclear experiments, medical areas, and accelerator and space physics studies, tho ...
* Hawking radiation
Hawking radiation is black-body radiation released outside a black hole's event horizon due to quantum effects according to a model developed by Stephen Hawking in 1974.
The radiation was not predicted by previous models which assumed that onc ...
* Ionizing radiation
Ionizing (ionising) radiation, including Radioactive decay, nuclear radiation, consists of subatomic particles or electromagnetic waves that have enough energy per individual photon or particle to ionization, ionize atoms or molecules by detaching ...
* Non-ionizing radiation
Non-ionizing (or non-ionising) radiation refers to any type of electromagnetic radiation that does not carry enough energy per quantum ( photon energy) to ionize atoms or molecules—that is, to completely remove an electron from an atom or mol ...
* Radiant energy
In physics, and in particular as measured by radiometry, radiant energy is the energy of electromagnetic radiation, electromagnetic and gravitational radiation. As energy, its SI unit is the joule (J). The quantity of radiant energy may be calcul ...
– radiation by a source into the surrounding environment.
* Radiation damage
Radiation damage is the effect of ionizing radiation on physical objects including non-living structural materials. It can be either detrimental or beneficial for materials.
Radiobiology is the study of the action of ionizing radiation on living ...
– adverse effects of ionizing radiation on materials and devices
* Radiation hardening
Radiation hardening is the process of making electronic components and circuits resistant to damage or malfunction caused by high levels of ionizing radiation (particle radiation and high-energy electromagnetic radiation), especially for environm ...
– making electronics resistant to failure in high ionizing radiation environments
* Radiation hormesis – ionizing radiation dosage threshold damage theory
* Radiation poisoning
Acute radiation syndrome (ARS), also known as radiation sickness or radiation poisoning, is a collection of health effects that are caused by being exposed to high amounts of ionizing radiation in a short period of time. Symptoms can start wit ...
– adverse effects of ionizing radiation on life forms
* Radiation properties
Thermal radiation is electromagnetic radiation emitted by the thermal motion of particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation. The emission of energy arises from a combination of electro ...
* Radiation Protection Convention, 1960 – by International Labour Organization
The International Labour Organization (ILO) is a United Nations agency whose mandate is to advance social and economic justice by setting international labour standards. Founded in October 1919 under the League of Nations, it is one of the firs ...
* Radioactive contamination
Radioactive contamination, also called radiological pollution, is the deposition of, or presence of Radioactive decay, radioactive substances on surfaces or within solids, liquids, or gases (including the human body), where their presence is uni ...
* Radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
Notes and references
External links
*Health Physics Society Public Education Website
from World Health Organization * ttps://www.bbc.com/news/health-12722435 Q&A: Health effects of radiation exposure ''BBC News'', 21 July 2011. * {{Authority control Physical phenomena