Quantum Systems on:
[Wikipedia]
[Google]
[Amazon]
 Quantum mechanics is the fundamental physical
Quantum mechanics is the fundamental physical
 Another consequence of the mathematical rules of quantum mechanics is the phenomenon of
Another consequence of the mathematical rules of quantum mechanics is the phenomenon of  Another non-classical phenomenon predicted by quantum mechanics is
Another non-classical phenomenon predicted by quantum mechanics is
 Some wave functions produce probability distributions that are independent of time, such as
Some wave functions produce probability distributions that are independent of time, such as
 The simplest example of a quantum system with a position degree of freedom is a free particle in a single spatial dimension. A free particle is one which is not subject to external influences, so that its Hamiltonian consists only of its kinetic energy:
The general solution of the Schrödinger equation is given by
which is a superposition of all possible plane waves , which are eigenstates of the momentum operator with momentum . The coefficients of the superposition are , which is the Fourier transform of the initial quantum state .
It is not possible for the solution to be a single momentum eigenstate, or a single position eigenstate, as these are not normalizable quantum states. Instead, we can consider a Gaussian wave packet:
which has Fourier transform, and therefore momentum distribution
We see that as we make smaller the spread in position gets smaller, but the spread in momentum gets larger. Conversely, by making larger we make the spread in momentum smaller, but the spread in position gets larger. This illustrates the uncertainty principle.
As we let the Gaussian wave packet evolve in time, we see that its center moves through space at a constant velocity (like a classical particle with no forces acting on it). However, the wave packet will also spread out as time progresses, which means that the position becomes more and more uncertain. The uncertainty in momentum, however, stays constant.
The simplest example of a quantum system with a position degree of freedom is a free particle in a single spatial dimension. A free particle is one which is not subject to external influences, so that its Hamiltonian consists only of its kinetic energy:
The general solution of the Schrödinger equation is given by
which is a superposition of all possible plane waves , which are eigenstates of the momentum operator with momentum . The coefficients of the superposition are , which is the Fourier transform of the initial quantum state .
It is not possible for the solution to be a single momentum eigenstate, or a single position eigenstate, as these are not normalizable quantum states. Instead, we can consider a Gaussian wave packet:
which has Fourier transform, and therefore momentum distribution
We see that as we make smaller the spread in position gets smaller, but the spread in momentum gets larger. Conversely, by making larger we make the spread in momentum smaller, but the spread in position gets larger. This illustrates the uncertainty principle.
As we let the Gaussian wave packet evolve in time, we see that its center moves through space at a constant velocity (like a classical particle with no forces acting on it). However, the wave packet will also spread out as time progresses, which means that the position becomes more and more uncertain. The uncertainty in momentum, however, stays constant.
 The particle in a one-dimensional potential energy box is the most mathematically simple example where restraints lead to the quantization of energy levels. The box is defined as having zero potential energy everywhere inside a certain region, and therefore infinite potential energy everywhere outside that region. For the one-dimensional case in the direction, the time-independent Schrödinger equation may be written
With the differential operator defined by
the previous equation is evocative of the Kinetic energy#Kinetic energy of rigid bodies, classic kinetic energy analogue,
with state in this case having energy coincident with the kinetic energy of the particle.
The general solutions of the Schrödinger equation for the particle in a box are
or, from Euler's formula,
The infinite potential walls of the box determine the values of and at and where must be zero. Thus, at ,
and . At ,
in which cannot be zero as this would conflict with the postulate that has norm 1. Therefore, since , must be an integer multiple of ,
This constraint on implies a constraint on the energy levels, yielding
A finite potential well is the generalization of the infinite potential well problem to potential wells having finite depth. The finite potential well problem is mathematically more complicated than the infinite particle-in-a-box problem as the wave function is not pinned to zero at the walls of the well. Instead, the wave function must satisfy more complicated mathematical boundary conditions as it is nonzero in regions outside the well. Another related problem is that of the rectangular potential barrier, which furnishes a model for the quantum tunneling effect that plays an important role in the performance of modern technologies such as flash memory and scanning tunneling microscopy.
The particle in a one-dimensional potential energy box is the most mathematically simple example where restraints lead to the quantization of energy levels. The box is defined as having zero potential energy everywhere inside a certain region, and therefore infinite potential energy everywhere outside that region. For the one-dimensional case in the direction, the time-independent Schrödinger equation may be written
With the differential operator defined by
the previous equation is evocative of the Kinetic energy#Kinetic energy of rigid bodies, classic kinetic energy analogue,
with state in this case having energy coincident with the kinetic energy of the particle.
The general solutions of the Schrödinger equation for the particle in a box are
or, from Euler's formula,
The infinite potential walls of the box determine the values of and at and where must be zero. Thus, at ,
and . At ,
in which cannot be zero as this would conflict with the postulate that has norm 1. Therefore, since , must be an integer multiple of ,
This constraint on implies a constraint on the energy levels, yielding
A finite potential well is the generalization of the infinite potential well problem to potential wells having finite depth. The finite potential well problem is mathematically more complicated than the infinite particle-in-a-box problem as the wave function is not pinned to zero at the walls of the well. Instead, the wave function must satisfy more complicated mathematical boundary conditions as it is nonzero in regions outside the well. Another related problem is that of the rectangular potential barrier, which furnishes a model for the quantum tunneling effect that plays an important role in the performance of modern technologies such as flash memory and scanning tunneling microscopy.
 As in the classical case, the potential for the quantum harmonic oscillator is given by
This problem can either be treated by directly solving the Schrödinger equation, which is not trivial, or by using the more elegant "ladder method" first proposed by Paul Dirac. The
As in the classical case, the potential for the quantum harmonic oscillator is given by
This problem can either be treated by directly solving the Schrödinger equation, which is not trivial, or by using the more elegant "ladder method" first proposed by Paul Dirac. The
 The Mach–Zehnder interferometer (MZI) illustrates the concepts of superposition and interference with linear algebra in dimension 2, rather than differential equations. It can be seen as a simplified version of the double-slit experiment, but it is of interest in its own right, for example in the delayed choice quantum eraser, the Elitzur–Vaidman bomb tester, and in studies of quantum entanglement.
We can model a photon going through the interferometer by considering that at each point it can be in a superposition of only two paths: the "lower" path which starts from the left, goes straight through both beam splitters, and ends at the top, and the "upper" path which starts from the bottom, goes straight through both beam splitters, and ends at the right. The quantum state of the photon is therefore a vector that is a superposition of the "lower" path and the "upper" path , that is, for complex . In order to respect the postulate that we require that .
Both beam splitters are modelled as the unitary matrix , which means that when a photon meets the beam splitter it will either stay on the same path with a probability amplitude of , or be reflected to the other path with a probability amplitude of . The phase shifter on the upper arm is modelled as the unitary matrix , which means that if the photon is on the "upper" path it will gain a relative phase of , and it will stay unchanged if it is in the lower path.
A photon that enters the interferometer from the left will then be acted upon with a beam splitter , a phase shifter , and another beam splitter , and so end up in the state
and the probabilities that it will be detected at the right or at the top are given respectively by
One can therefore use the Mach–Zehnder interferometer to estimate the Phase (waves), phase shift by estimating these probabilities.
It is interesting to consider what would happen if the photon were definitely in either the "lower" or "upper" paths between the beam splitters. This can be accomplished by blocking one of the paths, or equivalently by removing the first beam splitter (and feeding the photon from the left or the bottom, as desired). In both cases, there will be no interference between the paths anymore, and the probabilities are given by , independently of the phase . From this we can conclude that the photon does not take one path or another after the first beam splitter, but rather that it is in a genuine quantum superposition of the two paths.
The Mach–Zehnder interferometer (MZI) illustrates the concepts of superposition and interference with linear algebra in dimension 2, rather than differential equations. It can be seen as a simplified version of the double-slit experiment, but it is of interest in its own right, for example in the delayed choice quantum eraser, the Elitzur–Vaidman bomb tester, and in studies of quantum entanglement.
We can model a photon going through the interferometer by considering that at each point it can be in a superposition of only two paths: the "lower" path which starts from the left, goes straight through both beam splitters, and ends at the top, and the "upper" path which starts from the bottom, goes straight through both beam splitters, and ends at the right. The quantum state of the photon is therefore a vector that is a superposition of the "lower" path and the "upper" path , that is, for complex . In order to respect the postulate that we require that .
Both beam splitters are modelled as the unitary matrix , which means that when a photon meets the beam splitter it will either stay on the same path with a probability amplitude of , or be reflected to the other path with a probability amplitude of . The phase shifter on the upper arm is modelled as the unitary matrix , which means that if the photon is on the "upper" path it will gain a relative phase of , and it will stay unchanged if it is in the lower path.
A photon that enters the interferometer from the left will then be acted upon with a beam splitter , a phase shifter , and another beam splitter , and so end up in the state
and the probabilities that it will be detected at the right or at the top are given respectively by
One can therefore use the Mach–Zehnder interferometer to estimate the Phase (waves), phase shift by estimating these probabilities.
It is interesting to consider what would happen if the photon were definitely in either the "lower" or "upper" paths between the beam splitters. This can be accomplished by blocking one of the paths, or equivalently by removing the first beam splitter (and feeding the photon from the left or the bottom, as desired). In both cases, there will be no interference between the paths anymore, and the probabilities are given by , independently of the phase . From this we can conclude that the photon does not take one path or another after the first beam splitter, but rather that it is in a genuine quantum superposition of the two paths.
 Everett's
Everett's
 This phase is known as the
This phase is known as the
Draft of 4th edition.
*
Online copy
* * Gunther Ludwig, 1968. ''Wave Mechanics''. London: Pergamon Press. * George Mackey (2004). ''The mathematical foundations of quantum mechanics''. Dover Publications. . * * Albert Messiah, 1966. ''Quantum Mechanics'' (Vol. I), English translation from French by G. M. Temmer. North Holland, John Wiley & Sons. Cf. chpt. IV, section III
online
* * Considers the extent to which chemistry and the periodic system have been reduced to quantum mechanics. * * * * * Martinus J. G. Veltman, Veltman, Martinus J. G. (2003), ''Facts and Mysteries in Elementary Particle Physics''.
Introduction to Quantum Theory at Quantiki.
Quantum Physics Made Relatively Simple
three video lectures by Hans Bethe. Course material
Quantum Cook Book
an
PHYS 201: Fundamentals of Physics II
by Ramamurti Shankar, Yale OpenCourseware. *
Modern Physics: With waves, thermodynamics, and optics
' – an online textbook. * MIT OpenCourseWare
Chemistry
an
Physics
Se
8.04
an
Examples in Quantum Mechanics
Philosophy * * {{Authority control Quantum mechanics,
 Quantum mechanics is the fundamental physical
Quantum mechanics is the fundamental physical theory
A theory is a systematic and rational form of abstract thinking about a phenomenon, or the conclusions derived from such thinking. It involves contemplative and logical reasoning, often supported by processes such as observation, experimentation, ...
that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s. Reprinted, Addison-Wesley, 1989, It is the foundation of all quantum physics, which includes quantum chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
, quantum field theory
In theoretical physics, quantum field theory (QFT) is a theoretical framework that combines Field theory (physics), field theory and the principle of relativity with ideas behind quantum mechanics. QFT is used in particle physics to construct phy ...
, quantum technology
Quantum engineering is the development of technology that capitalizes on the laws of quantum mechanics. This type of engineering uses quantum mechanics to develop technologies such as quantum sensors and quantum computers.
Devices that rely on ...
, and quantum information science
Quantum information science is a field that combines the principles of quantum mechanics with information theory to study the processing, analysis, and transmission of information. It covers both theoretical and experimental aspects of quantum phys ...
.
Quantum mechanics can describe many systems that classical physics
Classical physics refers to physics theories that are non-quantum or both non-quantum and non-relativistic, depending on the context. In historical discussions, ''classical physics'' refers to pre-1900 physics, while '' modern physics'' refers to ...
cannot. Classical physics can describe many aspects of nature at an ordinary (macroscopic
The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments. It is the opposite of microscopic.
Overview
When applied to physical phenome ...
and (optical) microscopic) scale, but is not sufficient for describing them at very small submicroscopic
The table lists various objects and units by the order of magnitude of their volume.
Sub-microscopic
Microscopic
Human measures
Terrestrial
Astronomical
References
{{DEFAULTSORT:Orders Of Magnitude (Volume)
V ...
(atomic and subatomic
In physics, a subatomic particle is a particle smaller than an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles (for example, a baryon, like ...
) scales. Classical mechanics can be derived from quantum mechanics as an approximation that is valid at ordinary scales.
Quantum systems have bound
Bound or bounds may refer to:
Mathematics
* Bound variable
* Upper and lower bounds, observed limits of mathematical functions
Physics
* Bound state, a particle that has a tendency to remain localized in one or more regions of space
Geography
* ...
states that are quantized to discrete values of energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
, momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
, angular momentum
Angular momentum (sometimes called moment of momentum or rotational momentum) is the rotational analog of Momentum, linear momentum. It is an important physical quantity because it is a Conservation law, conserved quantity – the total ang ...
, and other quantities, in contrast to classical systems where these quantities can be measured continuously. Measurements of quantum systems show characteristics of both particle
In the physical sciences, a particle (or corpuscle in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from s ...
s and wave
In physics, mathematics, engineering, and related fields, a wave is a propagating dynamic disturbance (change from List of types of equilibrium, equilibrium) of one or more quantities. ''Periodic waves'' oscillate repeatedly about an equilibrium ...
s (wave–particle duality
Wave–particle duality is the concept in quantum mechanics that fundamental entities of the universe, like photons and electrons, exhibit particle or wave (physics), wave properties according to the experimental circumstances. It expresses the in ...
), and there are limits to how accurately the value of a physical quantity can be predicted prior to its measurement, given a complete set of initial conditions (the uncertainty principle
The uncertainty principle, also known as Heisenberg's indeterminacy principle, is a fundamental concept in quantum mechanics. It states that there is a limit to the precision with which certain pairs of physical properties, such as position a ...
).
Quantum mechanics arose gradually from theories to explain observations that could not be reconciled with classical physics
Classical physics refers to physics theories that are non-quantum or both non-quantum and non-relativistic, depending on the context. In historical discussions, ''classical physics'' refers to pre-1900 physics, while '' modern physics'' refers to ...
, such as Max Planck
Max Karl Ernst Ludwig Planck (; ; 23 April 1858 – 4 October 1947) was a German Theoretical physics, theoretical physicist whose discovery of energy quantum, quanta won him the Nobel Prize in Physics in 1918.
Planck made many substantial con ...
's solution in 1900 to the black-body radiation
Black-body radiation is the thermal radiation, thermal electromagnetic radiation within, or surrounding, a body in thermodynamic equilibrium with its environment, emitted by a black body (an idealized opaque, non-reflective body). It has a specific ...
problem, and the correspondence between energy and frequency in Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
's 1905 paper, which explained the photoelectric effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physi ...
. These early attempts to understand microscopic phenomena, now known as the "old quantum theory
The old quantum theory is a collection of results from the years 1900–1925, which predate modern quantum mechanics. The theory was never complete or self-consistent, but was instead a set of heuristic corrections to classical mechanics. The th ...
", led to the full development of quantum mechanics in the mid-1920s by Niels Bohr
Niels Henrik David Bohr (, ; ; 7 October 1885 – 18 November 1962) was a Danish theoretical physicist who made foundational contributions to understanding atomic structure and old quantum theory, quantum theory, for which he received the No ...
, Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger ( ; ; 12 August 1887 – 4 January 1961), sometimes written as or , was an Austrian-Irish theoretical physicist who developed fundamental results in quantum field theory, quantum theory. In particul ...
, Werner Heisenberg
Werner Karl Heisenberg (; ; 5 December 1901 – 1 February 1976) was a German theoretical physicist, one of the main pioneers of the theory of quantum mechanics and a principal scientist in the German nuclear program during World War II.
He pub ...
, Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German-British theoretical physicist who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics, and supervised the work of a ...
, Paul Dirac
Paul Adrien Maurice Dirac ( ; 8 August 1902 – 20 October 1984) was an English mathematician and Theoretical physics, theoretical physicist who is considered to be one of the founders of quantum mechanics. Dirac laid the foundations for bot ...
and others. The modern theory is formulated in various specially developed mathematical formalisms. In one of them, a mathematical entity called the wave function
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter) ...
provides information, in the form of probability amplitude
In quantum mechanics, a probability amplitude is a complex number used for describing the behaviour of systems. The square of the modulus of this quantity at a point in space represents a probability density at that point.
Probability amplitu ...
s, about what measurements of a particle's energy, momentum, and other physical properties may yield.
Overview and fundamental concepts
Quantum mechanics allows the calculation of properties and behaviour ofphysical systems
A physical system is a collection of physical objects under study. The collection differs from a set: all the objects must coexist and have some physical relationship.
In other words, it is a portion of the physical universe chosen for analysi ...
. It is typically applied to microscopic systems: molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
, atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
and subatomic particle
In physics, a subatomic particle is a particle smaller than an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles (for example, a baryon, lik ...
s. It has been demonstrated to hold for complex molecules with thousands of atoms, but its application to human beings raises philosophical problems, such as Wigner's friend
Wigner's friend is a thought experiment in theoretical quantum physics, first published by the Hungarian-American physicist Eugene Wigner in 1961, Reprinted in and further developed by David Deutsch in 1985. The scenario involves an indirect obse ...
, and its application to the universe as a whole remains speculative. Predictions of quantum mechanics have been verified experimentally to an extremely high degree of accuracy
Accuracy and precision are two measures of ''observational error''.
''Accuracy'' is how close a given set of measurements (observations or readings) are to their ''true value''.
''Precision'' is how close the measurements are to each other.
The ...
. For example, the refinement of quantum mechanics for the interaction of light and matter, known as quantum electrodynamics
In particle physics, quantum electrodynamics (QED) is the Theory of relativity, relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quant ...
(QED), has been shown to agree with experiment to within 1 part in 1012 when predicting the magnetic properties of an electron.
A fundamental feature of the theory is that it usually cannot predict with certainty what will happen, but only give probabilities. Mathematically, a probability is found by taking the square of the absolute value of a complex number
In mathematics, a complex number is an element of a number system that extends the real numbers with a specific element denoted , called the imaginary unit and satisfying the equation i^= -1; every complex number can be expressed in the for ...
, known as a probability amplitude. This is known as the Born rule
The Born rule is a postulate of quantum mechanics that gives the probability that a measurement of a quantum system will yield a given result. In one commonly used application, it states that the probability density for finding a particle at a ...
, named after physicist Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German-British theoretical physicist who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics, and supervised the work of a ...
. For example, a quantum particle like an electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
can be described by a wave function, which associates to each point in space a probability amplitude. Applying the Born rule to these amplitudes gives a probability density function
In probability theory, a probability density function (PDF), density function, or density of an absolutely continuous random variable, is a Function (mathematics), function whose value at any given sample (or point) in the sample space (the s ...
for the position that the electron will be found to have when an experiment is performed to measure it. This is the best the theory can do; it cannot say for certain where the electron will be found. The Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
relates the collection of probability amplitudes that pertain to one moment of time to the collection of probability amplitudes that pertain to another.
One consequence of the mathematical rules of quantum mechanics is a tradeoff in predictability between measurable quantities. The most famous form of this uncertainty principle
The uncertainty principle, also known as Heisenberg's indeterminacy principle, is a fundamental concept in quantum mechanics. It states that there is a limit to the precision with which certain pairs of physical properties, such as position a ...
says that no matter how a quantum particle is prepared or how carefully experiments upon it are arranged, it is impossible to have a precise prediction for a measurement of its position and also at the same time for a measurement of its momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
.
quantum interference
In physics, interference is a phenomenon in which two coherent waves are combined by adding their intensities or displacements with due consideration for their phase difference. The resultant wave may have greater amplitude (constructive int ...
, which is often illustrated with the double-slit experiment
In modern physics, the double-slit experiment demonstrates that light and matter can exhibit behavior of both classical particles and classical waves. This type of experiment was first performed by Thomas Young in 1801, as a demonstration of ...
. In the basic version of this experiment, a coherent light source, such as a laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
beam, illuminates a plate pierced by two parallel slits, and the light passing through the slits is observed on a screen behind the plate. The wave nature of light causes the light waves passing through the two slits to interfere, producing bright and dark bands on the screen – a result that would not be expected if light consisted of classical particles. However, the light is always found to be absorbed at the screen at discrete points, as individual particles rather than waves; the interference pattern appears via the varying density of these particle hits on the screen. Furthermore, versions of the experiment that include detectors at the slits find that each detected photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
passes through one slit (as would a classical particle), and not through both slits (as would a wave). However, such experiments demonstrate that particles do not form the interference pattern if one detects which slit they pass through. This behavior is known as wave–particle duality
Wave–particle duality is the concept in quantum mechanics that fundamental entities of the universe, like photons and electrons, exhibit particle or wave (physics), wave properties according to the experimental circumstances. It expresses the in ...
. In addition to light, electrons
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
, atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
, and molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
are all found to exhibit the same dual behavior when fired towards a double slit.
 Another non-classical phenomenon predicted by quantum mechanics is
Another non-classical phenomenon predicted by quantum mechanics is quantum tunnelling
In physics, quantum tunnelling, barrier penetration, or simply tunnelling is a quantum mechanical phenomenon in which an object such as an electron or atom passes through a potential energy barrier that, according to classical mechanics, shoul ...
: a particle that goes up against a potential barrier
In quantum mechanics, the rectangular (or, at times, square) potential barrier is a standard one-dimensional problem that demonstrates the phenomena of wave-mechanical tunneling (also called "quantum tunneling") and wave-mechanical reflection. ...
can cross it, even if its kinetic energy is smaller than the maximum of the potential. In classical mechanics this particle would be trapped. Quantum tunnelling has several important consequences, enabling radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
, nuclear fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the rele ...
in stars, and applications such as scanning tunnelling microscopy
A scanning tunneling microscope (STM) is a type of scanning probe microscope used for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer, then at IBM Zürich, the Nobel Prize in ...
, tunnel diode
A tunnel diode or Esaki diode is a type of semiconductor diode that has effectively " negative resistance" due to the quantum mechanical effect called tunneling. It was invented in August 1957 by Leo Esaki and Yuriko Kurose when working ...
and tunnel field-effect transistor
The tunnel field-effect transistor (TFET) is an experimental type of transistor. Even though its structure is very similar to a metal–oxide–semiconductor field-effect transistor (MOSFET), the fundamental switching mechanism differs, making this ...
.
When quantum systems interact, the result can be the creation of quantum entanglement
Quantum entanglement is the phenomenon where the quantum state of each Subatomic particle, particle in a group cannot be described independently of the state of the others, even when the particles are separated by a large distance. The topic o ...
: their properties become so intertwined that a description of the whole solely in terms of the individual parts is no longer possible. Erwin Schrödinger called entanglement "...the characteristic trait of quantum mechanics, the one that enforces its entire departure from classical lines of thought". Quantum entanglement enables quantum computing
A quantum computer is a computer that exploits quantum mechanical phenomena. On small scales, physical matter exhibits properties of wave-particle duality, both particles and waves, and quantum computing takes advantage of this behavior using s ...
and is part of quantum communication protocols, such as quantum key distribution
Quantum key distribution (QKD) is a secure communication method that implements a cryptographic protocol involving components of quantum mechanics. It enables two parties to produce a shared random secret key known only to them, which then can b ...
and superdense coding
In quantum information theory, superdense coding (also referred to as ''dense coding'') is a quantum communication protocol to communicate a number of classical bits of information by only transmitting a smaller number of qubits, under the ass ...
. Contrary to popular misconception, entanglement does not allow sending signals faster than light
Faster-than-light (superluminal or supercausal) travel and communication are the conjectural propagation of matter or information faster than the speed of light in vacuum (). The special theory of relativity implies that only particles with zero ...
, as demonstrated by the no-communication theorem
In physics, the no-communication theorem (also referred to as the no-signaling principle) is a no-go theorem in quantum information theory. It asserts that during the measurement of an entangled quantum state, it is impossible for one observer ...
.
Another possibility opened by entanglement is testing for " hidden variables", hypothetical properties more fundamental than the quantities addressed in quantum theory itself, knowledge of which would allow more exact predictions than quantum theory provides. A collection of results, most significantly Bell's theorem
Bell's theorem is a term encompassing a number of closely related results in physics, all of which determine that quantum mechanics is incompatible with local hidden-variable theories, given some basic assumptions about the nature of measuremen ...
, have demonstrated that broad classes of such hidden-variable theories are in fact incompatible with quantum physics. According to Bell's theorem, if nature actually operates in accord with any theory of local hidden variables, then the results of a Bell test
A Bell test, also known as Bell inequality test or Bell experiment, is a real-world physics experiment designed to test the theory of quantum mechanics in relation to Albert Einstein's concept of local realism. Named for John Stewart Bell, the exp ...
will be constrained in a particular, quantifiable way. Many Bell tests have been performed and they have shown results incompatible with the constraints imposed by local hidden variables.
It is not possible to present these concepts in more than a superficial way without introducing the mathematics involved; understanding quantum mechanics requires not only manipulating complex numbers, but also linear algebra
Linear algebra is the branch of mathematics concerning linear equations such as
:a_1x_1+\cdots +a_nx_n=b,
linear maps such as
:(x_1, \ldots, x_n) \mapsto a_1x_1+\cdots +a_nx_n,
and their representations in vector spaces and through matrix (mathemat ...
, differential equations, group theory
In abstract algebra, group theory studies the algebraic structures known as group (mathematics), groups.
The concept of a group is central to abstract algebra: other well-known algebraic structures, such as ring (mathematics), rings, field ( ...
, and other more advanced subjects. Accordingly, this article will present a mathematical formulation of quantum mechanics and survey its application to some useful and oft-studied examples.
Mathematical formulation
In the mathematically rigorous formulation of quantum mechanics, the state of a quantum mechanical system is a vector belonging to a ( separable) complexHilbert space
In mathematics, a Hilbert space is a real number, real or complex number, complex inner product space that is also a complete metric space with respect to the metric induced by the inner product. It generalizes the notion of Euclidean space. The ...
. This vector is postulated to be normalized under the Hilbert space inner product, that is, it obeys , and it is well-defined up to a complex number of modulus 1 (the global phase), that is, and represent the same physical system. In other words, the possible states are points in the projective space
In mathematics, the concept of a projective space originated from the visual effect of perspective, where parallel lines seem to meet ''at infinity''. A projective space may thus be viewed as the extension of a Euclidean space, or, more generally ...
of a Hilbert space, usually called the complex projective space
In mathematics, complex projective space is the projective space with respect to the field of complex numbers. By analogy, whereas the points of a real projective space label the lines through the origin of a real Euclidean space, the points of a ...
. The exact nature of this Hilbert space is dependent on the system – for example, for describing position and momentum the Hilbert space is the space of complex square-integrable
In mathematics, a square-integrable function, also called a quadratically integrable function or L^2 function or square-summable function, is a real- or complex-valued measurable function for which the integral of the square of the absolute value ...
functions , while the Hilbert space for the spin
Spin or spinning most often refers to:
* Spin (physics) or particle spin, a fundamental property of elementary particles
* Spin quantum number, a number which defines the value of a particle's spin
* Spinning (textiles), the creation of yarn or thr ...
of a single proton is simply the space of two-dimensional complex vectors with the usual inner product.
Physical quantities of interestposition, momentum, energy, spinare represented by observables, which are Hermitian {{Short description, none
Numerous things are named after the French mathematician Charles Hermite (1822–1901):
Hermite
* Cubic Hermite spline, a type of third-degree spline
* Gauss–Hermite quadrature, an extension of Gaussian quadrature me ...
(more precisely, self-adjoint
In mathematics, an element of a *-algebra is called self-adjoint if it is the same as its adjoint (i.e. a = a^*).
Definition
Let \mathcal be a *-algebra. An element a \in \mathcal is called self-adjoint if
The set of self-adjoint elements ...
) linear operators
Operator may refer to:
Mathematics
* A symbol indicating a mathematical operation
* Logical operator or logical connective in mathematical logic
* Operator (mathematics), mapping that acts on elements of a space to produce elements of another ...
acting on the Hilbert space. A quantum state can be an eigenvector
In linear algebra, an eigenvector ( ) or characteristic vector is a vector that has its direction unchanged (or reversed) by a given linear transformation. More precisely, an eigenvector \mathbf v of a linear transformation T is scaled by ...
of an observable, in which case it is called an eigenstate
In quantum physics, a quantum state is a mathematical entity that embodies the knowledge of a quantum system. Quantum mechanics specifies the construction, evolution, and measurement of a quantum state. The result is a prediction for the system re ...
, and the associated eigenvalue
In linear algebra, an eigenvector ( ) or characteristic vector is a vector that has its direction unchanged (or reversed) by a given linear transformation. More precisely, an eigenvector \mathbf v of a linear transformation T is scaled by a ...
corresponds to the value of the observable in that eigenstate. More generally, a quantum state will be a linear combination of the eigenstates, known as a quantum superposition
Quantum superposition is a fundamental principle of quantum mechanics that states that linear combinations of solutions to the Schrödinger equation are also solutions of the Schrödinger equation. This follows from the fact that the Schrödi ...
. When an observable is measured, the result will be one of its eigenvalues with probability given by the Born rule
The Born rule is a postulate of quantum mechanics that gives the probability that a measurement of a quantum system will yield a given result. In one commonly used application, it states that the probability density for finding a particle at a ...
: in the simplest case the eigenvalue is non-degenerate and the probability is given by , where is its associated unit-length eigenvector. More generally, the eigenvalue is degenerate and the probability is given by , where is the projector onto its associated eigenspace. In the continuous case, these formulas give instead the probability density
In probability theory, a probability density function (PDF), density function, or density of an absolutely continuous random variable, is a function whose value at any given sample (or point) in the sample space (the set of possible values ...
.
After the measurement, if result was obtained, the quantum state is postulated to collapse to , in the non-degenerate case, or to , in the general case. The probabilistic
Probability is a branch of mathematics and statistics concerning events and numerical descriptions of how likely they are to occur. The probability of an event is a number between 0 and 1; the larger the probability, the more likely an e ...
nature of quantum mechanics thus stems from the act of measurement. This is one of the most difficult aspects of quantum systems to understand. It was the central topic in the famous Bohr–Einstein debates, in which the two scientists attempted to clarify these fundamental principles by way of thought experiment
A thought experiment is an imaginary scenario that is meant to elucidate or test an argument or theory. It is often an experiment that would be hard, impossible, or unethical to actually perform. It can also be an abstract hypothetical that is ...
s. In the decades after the formulation of quantum mechanics, the question of what constitutes a "measurement" has been extensively studied. Newer interpretations of quantum mechanics
An interpretation of quantum mechanics is an attempt to explain how the mathematical theory of quantum mechanics might correspond to experienced reality. Quantum mechanics has held up to rigorous and extremely precise tests in an extraordinarily b ...
have been formulated that do away with the concept of "wave function collapse
In various interpretations of quantum mechanics, wave function collapse, also called reduction of the state vector, occurs when a wave function—initially in a superposition of several eigenstates—reduces to a single eigenstate due to in ...
" (see, for example, the many-worlds interpretation
The many-worlds interpretation (MWI) is an interpretation of quantum mechanics that asserts that the universal wavefunction is Philosophical realism, objectively real, and that there is no wave function collapse. This implies that all Possible ...
). The basic idea is that when a quantum system interacts with a measuring apparatus, their respective wave functions become entangled so that the original quantum system ceases to exist as an independent entity (see ''Measurement in quantum mechanics
In quantum physics, a measurement is the testing or manipulation of a physical system to yield a numerical result. A fundamental feature of quantum theory is that the predictions it makes are probabilistic. The procedure for finding a probability ...
'').
Time evolution of a quantum state
The time evolution of a quantum state is described by the Schrödinger equation: Here denotes theHamiltonian
Hamiltonian may refer to:
* Hamiltonian mechanics, a function that represents the total energy of a system
* Hamiltonian (quantum mechanics), an operator corresponding to the total energy of that system
** Dyall Hamiltonian, a modified Hamiltonian ...
, the observable corresponding to the total energy
Energy () is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat and light. Energy is a conserved quantity—the law of conservation of energy ...
of the system, and is the reduced Planck constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a ...
. The constant is introduced so that the Hamiltonian is reduced to the classical Hamiltonian in cases where the quantum system can be approximated by a classical system; the ability to make such an approximation in certain limits is called the correspondence principle
In physics, a correspondence principle is any one of several premises or assertions about the relationship between classical and quantum mechanics.
The physicist Niels Bohr coined the term in 1920 during the early development of quantum theory; ...
.
The solution of this differential equation is given by
The operator is known as the time-evolution operator, and has the crucial property that it is unitary
Unitary may refer to:
Mathematics
* Unitary divisor
* Unitary element
* Unitary group
* Unitary matrix
* Unitary morphism
* Unitary operator
* Unitary transformation
* Unitary representation
* Unitarity (physics)
* ''E''-unitary inverse semigr ...
. This time evolution is deterministic
Determinism is the metaphysical view that all events within the universe (or multiverse) can occur only in one possible way. Deterministic theories throughout the history of philosophy have developed from diverse and sometimes overlapping mo ...
in the sense that – given an initial quantum state – it makes a definite prediction of what the quantum state will be at any later time.
 Some wave functions produce probability distributions that are independent of time, such as
Some wave functions produce probability distributions that are independent of time, such as eigenstate
In quantum physics, a quantum state is a mathematical entity that embodies the knowledge of a quantum system. Quantum mechanics specifies the construction, evolution, and measurement of a quantum state. The result is a prediction for the system re ...
s of the Hamiltonian. Many systems that are treated dynamically in classical mechanics are described by such "static" wave functions. For example, a single electron in an unexcited atom is pictured classically as a particle moving in a circular trajectory around the atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the Department_of_Physics_and_Astronomy,_University_of_Manchester , University of Manchester ...
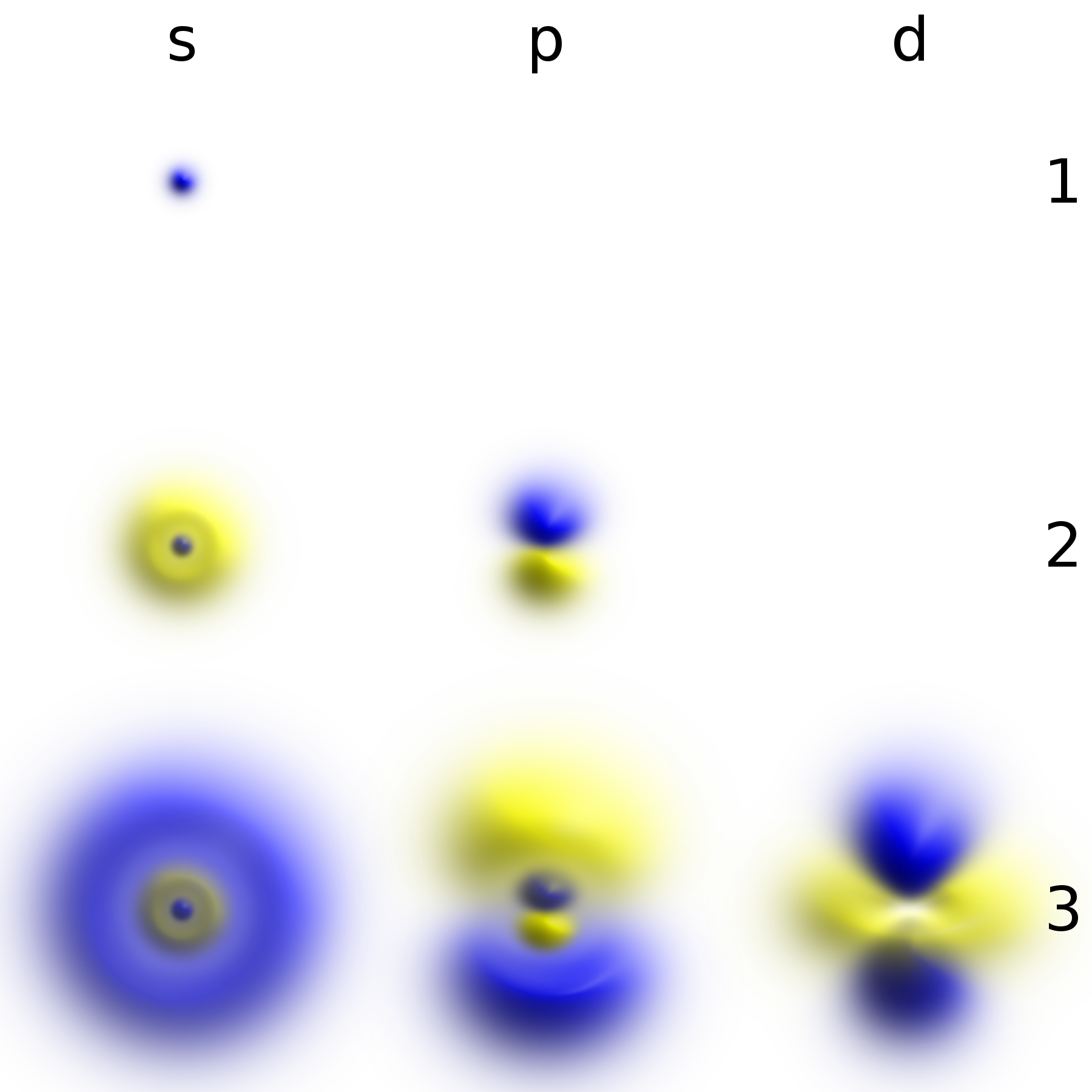
, whereas in quantum mechanics, it is described by a static wave function surrounding the nucleus. For example, the electron wave function for an unexcited hydrogen atom is a spherically symmetric function known as an ''s'' orbital ( Fig. 1).
Analytic solutions of the Schrödinger equation are known for very few relatively simple model Hamiltonians including the quantum harmonic oscillator
The quantum harmonic oscillator is the quantum-mechanical analog of the classical harmonic oscillator. Because an arbitrary smooth potential can usually be approximated as a harmonic potential at the vicinity of a stable equilibrium point, ...
, the particle in a box
In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes the movement of a free particle in a small space surrounded by impenetrable barriers. The model is mainly used a ...
, the dihydrogen cation
The dihydrogen cation or molecular hydrogen ion is a cation (positive ion) with formula H2^+. It consists of two hydrogen nuclei (protons), each sharing a single electron. It is the simplest molecular ion.
The ion can be formed from the ioniz ...
, and the hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral hydrogen atom contains a single positively charged proton in the nucleus, and a single negatively charged electron bound to the nucleus by the Coulomb for ...
. Even the helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
atom – which contains just two electrons – has defied all attempts at a fully analytic treatment, admitting no solution in closed form.
However, there are techniques for finding approximate solutions. One method, called perturbation theory
In mathematics and applied mathematics, perturbation theory comprises methods for finding an approximate solution to a problem, by starting from the exact solution of a related, simpler problem. A critical feature of the technique is a middle ...
, uses the analytic result for a simple quantum mechanical model to create a result for a related but more complicated model by (for example) the addition of a weak potential energy
In physics, potential energy is the energy of an object or system due to the body's position relative to other objects, or the configuration of its particles. The energy is equal to the work done against any restoring forces, such as gravity ...
. Another approximation method applies to systems for which quantum mechanics produces only small deviations from classical behavior. These deviations can then be computed based on the classical motion.
Uncertainty principle
One consequence of the basic quantum formalism is the uncertainty principle. In its most familiar form, this states that no preparation of a quantum particle can imply simultaneously precise predictions both for a measurement of its position and for a measurement of its momentum. Both position and momentum are observables, meaning that they are represented by Hermitian operators. The position operator and momentum operator do not commute, but rather satisfy thecanonical commutation relation
In quantum mechanics, the canonical commutation relation is the fundamental relation between canonical conjugate quantities (quantities which are related by definition such that one is the Fourier transform of another). For example,
hat x,\hat p ...
:
Given a quantum state, the Born rule lets us compute expectation values for both and , and moreover for powers of them. Defining the uncertainty for an observable by a standard deviation
In statistics, the standard deviation is a measure of the amount of variation of the values of a variable about its Expected value, mean. A low standard Deviation (statistics), deviation indicates that the values tend to be close to the mean ( ...
, we have
and likewise for the momentum:
The uncertainty principle states that
Either standard deviation can in principle be made arbitrarily small, but not both simultaneously.Section 3.2 of . This fact is experimentally well-known for example in quantum optics; see e.g. chap. 2 and Fig. 2.1 . This inequality generalizes to arbitrary pairs of self-adjoint operators and . The commutator
In mathematics, the commutator gives an indication of the extent to which a certain binary operation fails to be commutative. There are different definitions used in group theory and ring theory.
Group theory
The commutator of two elements, ...
of these two operators is
and this provides the lower bound on the product of standard deviations:
Another consequence of the canonical commutation relation is that the position and momentum operators are Fourier transform#Uncertainty principle, Fourier transforms of each other, so that a description of an object according to its momentum is the Fourier transform of its description according to its position. The fact that dependence in momentum is the Fourier transform of the dependence in position means that the momentum operator is equivalent (up to an factor) to taking the derivative according to the position, since in Fourier analysis Fourier transform#Differentiation, differentiation corresponds to multiplication in the dual space. This is why in quantum equations in position space, the momentum is replaced by , and in particular in the Schrödinger equation#Equation, non-relativistic Schrödinger equation in position space the momentum-squared term is replaced with a Laplacian times .
Composite systems and entanglement
When two different quantum systems are considered together, the Hilbert space of the combined system is the tensor product of the Hilbert spaces of the two components. For example, let and be two quantum systems, with Hilbert spaces and , respectively. The Hilbert space of the composite system is then If the state for the first system is the vector and the state for the second system is , then the state of the composite system is Not all states in the joint Hilbert space can be written in this form, however, because the superposition principle implies that linear combinations of these "separable" or "product states" are also valid. For example, if and are both possible states for system , and likewise and are both possible states for system , then is a valid joint state that is not separable. States that are not separable are called quantum entanglement, entangled. If the state for a composite system is entangled, it is impossible to describe either component system or system by a state vector. One can instead define reduced density matrix, reduced density matrices that describe the statistics that can be obtained by making measurements on either component system alone. This necessarily causes a loss of information, though: knowing the reduced density matrices of the individual systems is not enough to reconstruct the state of the composite system. Just as density matrices specify the state of a subsystem of a larger system, analogously, POVM, positive operator-valued measures (POVMs) describe the effect on a subsystem of a measurement performed on a larger system. POVMs are extensively used in quantum information theory. As described above, entanglement is a key feature of models of measurement processes in which an apparatus becomes entangled with the system being measured. Systems interacting with the environment in which they reside generally become entangled with that environment, a phenomenon known as quantum decoherence. This can explain why, in practice, quantum effects are difficult to observe in systems larger than microscopic.Equivalence between formulations
There are many mathematically equivalent formulations of quantum mechanics. One of the oldest and most common is the "transformation theory (quantum mechanics), transformation theory" proposed byPaul Dirac
Paul Adrien Maurice Dirac ( ; 8 August 1902 – 20 October 1984) was an English mathematician and Theoretical physics, theoretical physicist who is considered to be one of the founders of quantum mechanics. Dirac laid the foundations for bot ...
, which unifies and generalizes the two earliest formulations of quantum mechanics – matrix mechanics (invented by Werner Heisenberg
Werner Karl Heisenberg (; ; 5 December 1901 – 1 February 1976) was a German theoretical physicist, one of the main pioneers of the theory of quantum mechanics and a principal scientist in the German nuclear program during World War II.
He pub ...
) and wave mechanics (invented by Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger ( ; ; 12 August 1887 – 4 January 1961), sometimes written as or , was an Austrian-Irish theoretical physicist who developed fundamental results in quantum field theory, quantum theory. In particul ...
). An alternative formulation of quantum mechanics is Feynman's path integral formulation, in which a quantum-mechanical amplitude is considered as a sum over all possible classical and non-classical paths between the initial and final states. This is the quantum-mechanical counterpart of the action principle in classical mechanics.
Symmetries and conservation laws
The Hamiltonian is known as the ''generator'' of time evolution, since it defines a unitary time-evolution operator for each value of . From this relation between and , it follows that any observable that commutes with will be conserved: its expectation value will not change over time. This statement generalizes, as mathematically, any Hermitian operator can generate a family of unitary operators parameterized by a variable . Under the evolution generated by , any observable that commutes with will be conserved. Moreover, if is conserved by evolution under , then is conserved under the evolution generated by . This implies a quantum version of the result proven by Emmy Noether in classical (Lagrangian mechanics, Lagrangian) mechanics: for every differentiable Symmetry (physics), symmetry of a Hamiltonian, there exists a corresponding conservation law.Examples
Free particle
 The simplest example of a quantum system with a position degree of freedom is a free particle in a single spatial dimension. A free particle is one which is not subject to external influences, so that its Hamiltonian consists only of its kinetic energy:
The general solution of the Schrödinger equation is given by
which is a superposition of all possible plane waves , which are eigenstates of the momentum operator with momentum . The coefficients of the superposition are , which is the Fourier transform of the initial quantum state .
It is not possible for the solution to be a single momentum eigenstate, or a single position eigenstate, as these are not normalizable quantum states. Instead, we can consider a Gaussian wave packet:
which has Fourier transform, and therefore momentum distribution
We see that as we make smaller the spread in position gets smaller, but the spread in momentum gets larger. Conversely, by making larger we make the spread in momentum smaller, but the spread in position gets larger. This illustrates the uncertainty principle.
As we let the Gaussian wave packet evolve in time, we see that its center moves through space at a constant velocity (like a classical particle with no forces acting on it). However, the wave packet will also spread out as time progresses, which means that the position becomes more and more uncertain. The uncertainty in momentum, however, stays constant.
The simplest example of a quantum system with a position degree of freedom is a free particle in a single spatial dimension. A free particle is one which is not subject to external influences, so that its Hamiltonian consists only of its kinetic energy:
The general solution of the Schrödinger equation is given by
which is a superposition of all possible plane waves , which are eigenstates of the momentum operator with momentum . The coefficients of the superposition are , which is the Fourier transform of the initial quantum state .
It is not possible for the solution to be a single momentum eigenstate, or a single position eigenstate, as these are not normalizable quantum states. Instead, we can consider a Gaussian wave packet:
which has Fourier transform, and therefore momentum distribution
We see that as we make smaller the spread in position gets smaller, but the spread in momentum gets larger. Conversely, by making larger we make the spread in momentum smaller, but the spread in position gets larger. This illustrates the uncertainty principle.
As we let the Gaussian wave packet evolve in time, we see that its center moves through space at a constant velocity (like a classical particle with no forces acting on it). However, the wave packet will also spread out as time progresses, which means that the position becomes more and more uncertain. The uncertainty in momentum, however, stays constant.
Particle in a box
Harmonic oscillator
 As in the classical case, the potential for the quantum harmonic oscillator is given by
This problem can either be treated by directly solving the Schrödinger equation, which is not trivial, or by using the more elegant "ladder method" first proposed by Paul Dirac. The
As in the classical case, the potential for the quantum harmonic oscillator is given by
This problem can either be treated by directly solving the Schrödinger equation, which is not trivial, or by using the more elegant "ladder method" first proposed by Paul Dirac. The eigenstate
In quantum physics, a quantum state is a mathematical entity that embodies the knowledge of a quantum system. Quantum mechanics specifies the construction, evolution, and measurement of a quantum state. The result is a prediction for the system re ...
s are given by
where ''Hn'' are the Hermite polynomials
and the corresponding energy levels are
This is another example illustrating the discretization of energy for bound states.
Mach–Zehnder interferometer
Applications
Quantum mechanics has had enormous success in explaining many of the features of our universe, with regard to small-scale and discrete quantities and interactions which cannot be explained by Classical physics, classical methods. Quantum mechanics is often the only theory that can reveal the individual behaviors of the subatomic particles that make up all forms of matter (electrons, protons, neutrons,photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s, and others). Solid-state physics and materials science are dependent upon quantum mechanics.
In many aspects, modern technology operates at a scale where quantum effects are significant. Important applications of quantum theory include quantum chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
, quantum optics, quantum computing
A quantum computer is a computer that exploits quantum mechanical phenomena. On small scales, physical matter exhibits properties of wave-particle duality, both particles and waves, and quantum computing takes advantage of this behavior using s ...
, superconducting magnets, light-emitting diodes, the optical amplifier and the laser, the transistor and semiconductors such as the microprocessor, medical imaging, medical and research imaging such as magnetic resonance imaging and electron microscopy. Explanations for many biological and physical phenomena are rooted in the nature of the chemical bond, most notably the macro-molecule DNA.
Relation to other scientific theories
Classical mechanics
The rules of quantum mechanics assert that the state space of a system is a Hilbert space and that observables of the system are Hermitian operators acting on vectors in that space – although they do not tell us which Hilbert space or which operators. These can be chosen appropriately in order to obtain a quantitative description of a quantum system, a necessary step in making physical predictions. An important guide for making these choices is thecorrespondence principle
In physics, a correspondence principle is any one of several premises or assertions about the relationship between classical and quantum mechanics.
The physicist Niels Bohr coined the term in 1920 during the early development of quantum theory; ...
, a heuristic which states that the predictions of quantum mechanics reduce to those of classical mechanics in the regime of large quantum numbers. One can also start from an established classical model of a particular system, and then try to guess the underlying quantum model that would give rise to the classical model in the correspondence limit. This approach is known as Canonical quantization, quantization.
When quantum mechanics was originally formulated, it was applied to models whose correspondence limit was theory of relativity, non-relativistic classical mechanics. For instance, the well-known model of the quantum harmonic oscillator
The quantum harmonic oscillator is the quantum-mechanical analog of the classical harmonic oscillator. Because an arbitrary smooth potential can usually be approximated as a harmonic potential at the vicinity of a stable equilibrium point, ...
uses an explicitly non-relativistic expression for the kinetic energy of the oscillator, and is thus a quantum version of the harmonic oscillator, classical harmonic oscillator.
Complications arise with chaotic systems, which do not have good quantum numbers, and quantum chaos studies the relationship between classical and quantum descriptions in these systems.
Quantum decoherence is a mechanism through which quantum systems lose quantum coherence, coherence, and thus become incapable of displaying many typically quantum effects: quantum superposition
Quantum superposition is a fundamental principle of quantum mechanics that states that linear combinations of solutions to the Schrödinger equation are also solutions of the Schrödinger equation. This follows from the fact that the Schrödi ...
s become simply probabilistic mixtures, and quantum entanglement becomes simply classical correlations. Quantum coherence is not typically evident at macroscopic scales, though at temperatures approaching absolute zero quantum behavior may manifest macroscopically.
Many macroscopic properties of a classical system are a direct consequence of the quantum behavior of its parts. For example, the stability of bulk matter (consisting of atoms and molecules which would quickly collapse under electric forces alone), the rigidity of solids, and the mechanical, thermal, chemical, optical and magnetic properties of matter are all results of the interaction of electric charges under the rules of quantum mechanics.
Special relativity and electrodynamics
Early attempts to merge quantum mechanics with special relativity involved the replacement of the Schrödinger equation with a covariant equation such as the Klein–Gordon equation or the Dirac equation. While these theories were successful in explaining many experimental results, they had certain unsatisfactory qualities stemming from their neglect of the relativistic creation and annihilation of particles. A fully relativistic quantum theory required the development of quantum field theory, which applies quantization to a field (rather than a fixed set of particles). The first complete quantum field theory,quantum electrodynamics
In particle physics, quantum electrodynamics (QED) is the Theory of relativity, relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quant ...
, provides a fully quantum description of the electromagnetic interaction. Quantum electrodynamics is, along with general relativity, one of the most accurate physical theories ever devised.
The full apparatus of quantum field theory is often unnecessary for describing electrodynamic systems. A simpler approach, one that has been used since the inception of quantum mechanics, is to treat electric charge, charged particles as quantum mechanical objects being acted on by a classical electromagnetic field. For example, the elementary quantum model of the hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral hydrogen atom contains a single positively charged proton in the nucleus, and a single negatively charged electron bound to the nucleus by the Coulomb for ...
describes the electric field of the hydrogen atom using a classical Electric potential, Coulomb potential. Likewise, in a Stern–Gerlach experiment, a charged particle is modeled as a quantum system, while the background magnetic field is described classically. This "semi-classical" approach fails if quantum fluctuations in the electromagnetic field play an important role, such as in the emission of photons by charged particles.
Field (physics), Quantum field theories for the strong nuclear force and the weak nuclear force have also been developed. The quantum field theory of the strong nuclear force is called quantum chromodynamics, and describes the interactions of subnuclear particles such as quarks and gluons. The weak nuclear force and the electromagnetic force were unified, in their quantized forms, into a single quantum field theory (known as electroweak theory), by the physicists Abdus Salam, Sheldon Glashow and Steven Weinberg.
Relation to general relativity
Even though the predictions of both quantum theory and general relativity have been supported by rigorous and repeated empirical evidence, their abstract formalisms contradict each other and they have proven extremely difficult to incorporate into one consistent, cohesive model. Gravity is negligible in many areas of particle physics, so that unification between general relativity and quantum mechanics is not an urgent issue in those particular applications. However, the lack of a correct theory of quantum gravity is an important issue in physical cosmology and the search by physicists for an elegant "Theory of Everything" (TOE). Consequently, resolving the inconsistencies between both theories has been a major goal of 20th- and 21st-century physics. This TOE would combine not only the models of subatomic physics but also derive the four fundamental forces of nature from a single force or phenomenon. One proposal for doing so is string theory, which posits that the Point particle, point-like particles of particle physics are replaced by Dimension (mathematics and physics), one-dimensional objects called String (physics), strings. String theory describes how these strings propagate through space and interact with each other. On distance scales larger than the string scale, a string looks just like an ordinary particle, with its mass, charge (physics), charge, and other properties determined by the vibrational state of the string. In string theory, one of the many vibrational states of the string corresponds to the graviton, a quantum mechanical particle that carries gravitational force. Another popular theory is loop quantum gravity (LQG), which describes quantum properties of gravity and is thus a theory of quantum spacetime. LQG is an attempt to merge and adapt standard quantum mechanics and standard general relativity. This theory describes space as an extremely fine fabric "woven" of finite loops called spin networks. The evolution of a spin network over time is called a spin foam. The characteristic length scale of a spin foam is the Planck length, approximately 1.616×10−35 m, and so lengths shorter than the Planck length are not physically meaningful in LQG.Philosophical implications
Since its inception, the many counter-intuitive aspects and results of quantum mechanics have provoked strong philosophical debates and many interpretations of quantum mechanics, interpretations. The arguments centre on the probabilistic nature of quantum mechanics, the difficulties with wavefunction collapse and the related measurement problem, and quantum nonlocality. Perhaps the only consensus that exists about these issues is that there is no consensus. Richard Feynman once said, "I think I can safely say that nobody understands quantum mechanics." According to Steven Weinberg, "There is now in my opinion no entirely satisfactory interpretation of quantum mechanics." The views ofNiels Bohr
Niels Henrik David Bohr (, ; ; 7 October 1885 – 18 November 1962) was a Danish theoretical physicist who made foundational contributions to understanding atomic structure and old quantum theory, quantum theory, for which he received the No ...
, Werner Heisenberg and other physicists are often grouped together as the "Copenhagen interpretation". According to these views, the probabilistic nature of quantum mechanics is not a temporary feature which will eventually be replaced by a deterministic theory, but is instead a final renunciation of the classical idea of "causality". Bohr in particular emphasized that any well-defined application of the quantum mechanical formalism must always make reference to the experimental arrangement, due to the complementarity (physics), complementary nature of evidence obtained under different experimental situations. Copenhagen-type interpretations were adopted by Nobel laureates in quantum physics, including Bohr, Heisenberg, Schrödinger, Feynman, and Anton Zeilinger, Zeilinger as well as 21st-century researchers in quantum foundations.
Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
, himself one of the founders of Old quantum theory, quantum theory, was troubled by its apparent failure to respect some cherished metaphysical principles, such as determinism and principle of locality, locality. Einstein's long-running exchanges with Bohr about the meaning and status of quantum mechanics are now known as the Bohr–Einstein debates. Einstein believed that underlying quantum mechanics must be a theory that explicitly forbids action at a distance. He argued that quantum mechanics was incomplete, a theory that was valid but not fundamental, analogous to how thermodynamics is valid, but the fundamental theory behind it is statistical mechanics. In 1935, Einstein and his collaborators Boris Podolsky and Nathan Rosen published an argument that the principle of locality implies the incompleteness of quantum mechanics, a thought experiment
A thought experiment is an imaginary scenario that is meant to elucidate or test an argument or theory. It is often an experiment that would be hard, impossible, or unethical to actually perform. It can also be an abstract hypothetical that is ...
later termed the Einstein–Podolsky–Rosen paradox. In 1964, John Stewart Bell, John Bell showed that EPR's principle of locality, together with determinism, was actually incompatible with quantum mechanics: they implied constraints on the correlations produced by distance systems, now known as Bell inequalities, that can be violated by entangled particles. Since then Bell test, several experiments have been performed to obtain these correlations, with the result that they do in fact violate Bell inequalities, and thus falsify the conjunction of locality with determinism.
Bohmian mechanics shows that it is possible to reformulate quantum mechanics to make it deterministic, at the price of making it explicitly nonlocal. It attributes not only a wave function to a physical system, but in addition a real position, that evolves deterministically under a nonlocal guiding equation. The evolution of a physical system is given at all times by the Schrödinger equation together with the guiding equation; there is never a collapse of the wave function. This solves the measurement problem.
many-worlds interpretation
The many-worlds interpretation (MWI) is an interpretation of quantum mechanics that asserts that the universal wavefunction is Philosophical realism, objectively real, and that there is no wave function collapse. This implies that all Possible ...
, formulated in 1956, holds that all the possibilities described by quantum theory simultaneously occur in a multiverse composed of mostly independent parallel universes. This is a consequence of removing the axiom of the collapse of the wave packet. All possible states of the measured system and the measuring apparatus, together with the observer, are present in a real physical quantum superposition. While the multiverse is deterministic, we perceive non-deterministic behavior governed by probabilities, because we do not observe the multiverse as a whole, but only one parallel universe at a time. Exactly how this is supposed to work has been the subject of much debate. Several attempts have been made to make sense of this and derive the Born rule, with no consensus on whether they have been successful.
Relational quantum mechanics appeared in the late 1990s as a modern derivative of Copenhagen-type ideas, and QBism was developed some years later.
History
Quantum mechanics was developed in the early decades of the 20th century, driven by the need to explain phenomena that, in some cases, had been observed in earlier times. Scientific inquiry into the wave nature of light began in the 17th and 18th centuries, when scientists such as Robert Hooke, Christiaan Huygens and Leonhard Euler proposed a wave theory of light based on experimental observations. In 1803 English polymath Thomas Young (scientist), Thomas Young described the famous Young's interference experiment, double-slit experiment. This experiment played a major role in the general acceptance of the wave theory of light. During the early 19th century, chemistry, chemical research by John Dalton and Amedeo Avogadro lent weight to the atomic theory of matter, an idea that James Clerk Maxwell, Ludwig Boltzmann and others built upon to establish the kinetic theory of gases. The successes of kinetic theory gave further credence to the idea that matter is composed of atoms, yet the theory also had shortcomings that would only be resolved by the development of quantum mechanics. Reprinted, Addison-Wesley, 1989, While the early conception of atoms from Greek philosophy had been that they were indivisible unitsthe word "atom" deriving from the Greek language, Greek for 'uncuttable'the 19th century saw the formulation of hypotheses about subatomic structure. One important discovery in that regard was Michael Faraday's 1838 observation of a glow caused by an electrical discharge inside a glass tube containing gas at low pressure. Julius Plücker, Johann Wilhelm Hittorf and Eugen Goldstein carried on and improved upon Faraday's work, leading to the identification of cathode rays, which J. J. Thomson found to consist of subatomic particles that would be called electrons. Theblack-body radiation
Black-body radiation is the thermal radiation, thermal electromagnetic radiation within, or surrounding, a body in thermodynamic equilibrium with its environment, emitted by a black body (an idealized opaque, non-reflective body). It has a specific ...
problem was discovered by Gustav Kirchhoff in 1859. In 1900, Max Planck proposed the hypothesis that energy is radiated and absorbed in discrete "quanta" (or energy packets), yielding a calculation that precisely matched the observed patterns of black-body radiation. The word ''quantum'' derives from the Latin, meaning "how great" or "how much". According to Planck, quantities of energy could be thought of as divided into "elements" whose size (''E'') would be proportional to their frequency (''ν''):
,
where ''h'' is the Planck constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a ...
. Planck cautiously insisted that this was only an aspect of the processes of absorption and emission of radiation and was not the physical reality of the radiation. In fact, he considered his quantum hypothesis a mathematical trick to get the right answer rather than a sizable discovery. However, in 1905 Albert Einstein interpreted Planck's quantum hypothesis local realism, realistically and used it to explain the photoelectric effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physi ...
, in which shining light on certain materials can eject electrons from the material. Niels Bohr then developed Planck's ideas about radiation into a Bohr model, model of the hydrogen atom that successfully predicted the spectral lines of hydrogen. Einstein further developed this idea to show that an electromagnetic wave such as light could also be described as a particle (later called the photon), with a discrete amount of energy that depends on its frequency. In his paper "On the Quantum Theory of Radiation", Einstein expanded on the interaction between energy and matter to explain the absorption and emission of energy by atoms. Although overshadowed at the time by his general theory of relativity, this paper articulated the mechanism underlying the stimulated emission of radiation, which became the basis of the laser.
 This phase is known as the
This phase is known as the old quantum theory
The old quantum theory is a collection of results from the years 1900–1925, which predate modern quantum mechanics. The theory was never complete or self-consistent, but was instead a set of heuristic corrections to classical mechanics. The th ...
. Never complete or self-consistent, the old quantum theory was rather a set of heuristic corrections to classical mechanics. The theory is now understood as a WKB approximation#Application to the Schrödinger equation, semi-classical approximation to modern quantum mechanics. Notable results from this period include, in addition to the work of Planck, Einstein and Bohr mentioned above, Einstein and Peter Debye's work on the specific heat of solids, Bohr and Hendrika Johanna van Leeuwen's Bohr–Van Leeuwen theorem, proof that classical physics cannot account for diamagnetism, and Arnold Sommerfeld's extension of the Bohr model to include special-relativistic effects.
In the mid-1920s quantum mechanics was developed to become the standard formulation for atomic physics. In 1923, the French physicist Louis de Broglie put forward his theory of matter waves by stating that particles can exhibit wave characteristics and vice versa. Building on de Broglie's approach, modern quantum mechanics was born in 1925, when the German physicists Werner Heisenberg, Max Born, and Pascual JordanDavid Edwards, "The Mathematical Foundations of Quantum Mechanics", ''Synthese'', Volume 42, Number 1/September, 1979, pp. 1–70.David Edwards, "The Mathematical Foundations of Quantum Field Theory: Fermions, Gauge Fields, and Super-symmetry, Part I: Lattice Field Theories", ''International Journal of Theoretical Physics'', Vol. 20, No. 7 (1981). developed matrix mechanics and the Austrian physicist Erwin Schrödinger invented Schrödinger equation, wave mechanics. Born introduced the probabilistic interpretation of Schrödinger's wave function in July 1926. Thus, the entire field of quantum physics emerged, leading to its wider acceptance at the Fifth Solvay Conference in 1927.
By 1930, quantum mechanics had been further unified and formalized by David Hilbert, Paul Dirac and John von Neumann with greater emphasis on measurement in quantum mechanics, measurement, the statistical nature of our knowledge of reality, and Interpretations of quantum mechanics, philosophical speculation about the 'observer'. It has since permeated many disciplines, including quantum chemistry, quantum electronics, quantum optics, and quantum information science
Quantum information science is a field that combines the principles of quantum mechanics with information theory to study the processing, analysis, and transmission of information. It covers both theoretical and experimental aspects of quantum phys ...
. It also provides a useful framework for many features of the modern periodic table of elements, and describes the behaviors of atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
during chemical bonding and the flow of electrons in computer semiconductors, and therefore plays a crucial role in many modern technologies. While quantum mechanics was constructed to describe the world of the very small, it is also needed to explain some macroscopic
The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments. It is the opposite of microscopic.
Overview
When applied to physical phenome ...
phenomena such as superconductors and superfluids.
See also
* Bra–ket notation * Einstein's thought experiments * List of textbooks on classical mechanics and quantum mechanics * Macroscopic quantum phenomena * Phase-space formulation * Regularization (physics) * Two-state quantum systemExplanatory notes
References
Further reading
The following titles, all by working physicists, attempt to communicate quantum theory to lay people, using a minimum of technical apparatus: * Marvin Chester, Chester, Marvin (1987). ''Primer of Quantum Mechanics''. John Wiley. * * Richard Feynman, 1985. ''QED: The Strange Theory of Light and Matter'', Princeton University Press. . Four elementary lectures on quantum electrodynamics andquantum field theory
In theoretical physics, quantum field theory (QFT) is a theoretical framework that combines Field theory (physics), field theory and the principle of relativity with ideas behind quantum mechanics. QFT is used in particle physics to construct phy ...
, yet containing many insights for the expert.
* Giancarlo Ghirardi, Ghirardi, GianCarlo, 2004. ''Sneaking a Look at God's Cards'', Gerald Malsbary, trans. Princeton Univ. Press. The most technical of the works cited here. Passages using algebra, trigonometry, and bra–ket notation can be passed over on a first reading.
* N. David Mermin, 1990, "Spooky actions at a distance: mysteries of the QT" in his ''Boojums All the Way Through''. Cambridge University Press: 110–76.
* Victor Stenger, 2000. ''Timeless Reality: Symmetry, Simplicity, and Multiple Universes''. Buffalo, New York: Prometheus Books. Chpts. 5–8. Includes cosmological and philosophical considerations.
More technical:
*
*
*
*
* Bryce DeWitt, R. Neill Graham, eds., 1973. ''The Many-Worlds Interpretation of Quantum Mechanics'', Princeton Series in Physics, Princeton University Press.
*
*
* Daniel Greenberger, D. Greenberger, Klaus Hentschel, K. Hentschel, F. Weinert, eds., 2009. ''Compendium of quantum physics, Concepts, experiments, history and philosophy'', Springer-Verlag, Berlin, Heidelberg. Short articles on many QM topics.
* A standard undergraduate text.
* Max Jammer, 1966. ''The Conceptual Development of Quantum Mechanics''. McGraw Hill.
* Hagen Kleinert, 2004. ''Path Integrals in Quantum Mechanics, Statistics, Polymer Physics, and Financial Markets'', 3rd ed. Singapore: World ScientificDraft of 4th edition.
*
Online copy
* * Gunther Ludwig, 1968. ''Wave Mechanics''. London: Pergamon Press. * George Mackey (2004). ''The mathematical foundations of quantum mechanics''. Dover Publications. . * * Albert Messiah, 1966. ''Quantum Mechanics'' (Vol. I), English translation from French by G. M. Temmer. North Holland, John Wiley & Sons. Cf. chpt. IV, section III
online
* * Considers the extent to which chemistry and the periodic system have been reduced to quantum mechanics. * * * * * Martinus J. G. Veltman, Veltman, Martinus J. G. (2003), ''Facts and Mysteries in Elementary Particle Physics''.
External links
Introduction to Quantum Theory at Quantiki.
Quantum Physics Made Relatively Simple
three video lectures by Hans Bethe. Course material
Quantum Cook Book
an
PHYS 201: Fundamentals of Physics II
by Ramamurti Shankar, Yale OpenCourseware. *
Modern Physics: With waves, thermodynamics, and optics
' – an online textbook. * MIT OpenCourseWare
Chemistry
an
Physics
Se
8.04
an
Examples in Quantum Mechanics
Philosophy * * {{Authority control Quantum mechanics,