Pyramidal Carbocation on:
[Wikipedia]
[Google]
[Amazon]
 A pyramidal carbocation is a type of
A pyramidal carbocation is a type of
 : 1a starting situation in the calculations: the chloride ion just left.
: 1b the expected structure. Charge has been delocalized over three carbon atoms
: 1c representation of the pyramidal ion.
Depending on the method used, the ion 1c in figure 1 is an absolute or just a relative minimum.
: 1a starting situation in the calculations: the chloride ion just left.
: 1b the expected structure. Charge has been delocalized over three carbon atoms
: 1c representation of the pyramidal ion.
Depending on the method used, the ion 1c in figure 1 is an absolute or just a relative minimum.
The group "R" is either 1H or 2H ( D):
3HO-1,4-diMe-tricyclo(2,1,0,02,5)pentaan.jpg, 4a:
3-hydroxy-1,4-dimethyl-TCP 3HO-1,5-diMe-tricyclo(2,1,0,02,5)pentaan.jpg, 4b:
3-hydroxy-1,5-dimethyl-TCP 3MeO-3,5-diMe-tricyclo(2,1,0,02,5)pentaan.jpg, 4c:
3-methoxy-3,5-dimethyl-TCP Four sided Pyramidal 1,5-DiMe-3-R.jpg, 4d:
resulting carbocation
In 1972 Masamune describes the results of dissolving a number of precursors to ''4d'' (figure 4) at in
The "R"-group is either1H or 2H ( D):
pyramidal ion 4 sided with numbers.jpg , 5a = 4d
Pyramidal cation 3BzO-1,4-diMe-tricyclo(2,1,0,02,5)pentaan.jpg, 5b
Reaction product with benzoic acid
in dipolar aprotic solvent 3MeO-3,5-diMe-tricyclo(2,1,0,02,5)pentaan.jpg, 5c
reaction product with methanol
in methanol
As described above, independent from its synthetic route, pyramidal ion 5a reacts with methanol or benzoate giving rise to products governed by reagent and the reaction medium as is clear by the substitution patterns. In 1972 Masamune is unable to explain the different behavior of the intermediate. In terms of the HSAB-theory an explanation might be given.
In 1975 Masamune calculated in the non-substituted ion most of the charge at the hydrogen atoms. Replacing hydrogen for carbon, the central atom of the methyl group, a more
Pyramidal ion 4 sided Bishomo bridged.jpg , Bridges between the homo-atoms bishomo-carbocation
Pyramidal ion 4 sided Bishomo Unbridged with planes.jpg , The planes containing the sp2 hybrised atoms and those atoms connected to them
Pyramidal ion 4 sided Bishomo Unbridged.jpg , Unbridges bishomo-carbocation
The stability of this ion at first may seem strange, as enlargement of the ring in general will diminish the bonding overlap between the orbitals at the center of the pyramidal structure. Here the sp2 hybridization, and consequently the planarity of the atoms of and those directly bonded to the sp2 centers, forces the tops of the p-orbitals of the basal carbons towards each other, thus creating a solid base for the apical carbon to sit on. Stiffening the configuration by a bridge between the homo-atoms, converting the base of the pyramid, to a
 A pyramidal carbocation is a type of
A pyramidal carbocation is a type of carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
with a specific configuration. This ion exists as a third class, besides the classical and non-classical ions. In these ions, a single carbon atom hovers over a four- or five-sided polygon
In geometry, a polygon () is a plane figure made up of line segments connected to form a closed polygonal chain.
The segments of a closed polygonal chain are called its '' edges'' or ''sides''. The points where two edges meet are the polygon ...
, in effect forming a pyramid
A pyramid () is a structure whose visible surfaces are triangular in broad outline and converge toward the top, making the appearance roughly a pyramid in the geometric sense. The base of a pyramid can be of any polygon shape, such as trian ...
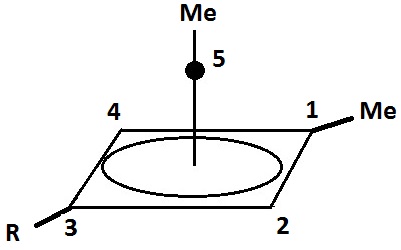
. The four-sided pyramidal ion will carry a charge of 1+, and the five-sided pyramid will carry 2+. In the images (''at upper right)'', the black spot on the vertical line represents the hovering carbon atom.
The apparent coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
of five, or even six, associated with the carbon atom at the top of the pyramid is a rarity as compared to the usual maximum of four.
History
Studying these cations was sparked, at the time, by amazing results incomputational chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of mol ...
. While calculating the optimal geometry of the mono-cation which arises from the extraction of chloride from 3-chlorotricyclo .1.0.02,5entane, the three bridges were expected to orient in space with angles of roughly 120°. The calculations however showed the four-sided pyramid to be the most stable configuration. At the top of this pyramid, there resides a carbon atom, still connected to a hydrogen. The original expected structure turned out to be not even close to an energy minimum: it represented a maximum.
:Figure 1: Several possibilities for (CH)5 cation.
: : 1a starting situation in the calculations: the chloride ion just left.
: 1b the expected structure. Charge has been delocalized over three carbon atoms
: 1c representation of the pyramidal ion.
Depending on the method used, the ion 1c in figure 1 is an absolute or just a relative minimum.
: 1a starting situation in the calculations: the chloride ion just left.
: 1b the expected structure. Charge has been delocalized over three carbon atoms
: 1c representation of the pyramidal ion.
Depending on the method used, the ion 1c in figure 1 is an absolute or just a relative minimum.
Theoretical background
A complete theoretical discussion will use all orbitals of all contributing atoms. A first approximation might use aLCAO
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefu ...
of the molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
s in the polygon
In geometry, a polygon () is a plane figure made up of line segments connected to form a closed polygonal chain.
The segments of a closed polygonal chain are called its '' edges'' or ''sides''. The points where two edges meet are the polygon ...
forming the base of the pyramid and the orbitals on the apical
Apical means "pertaining to an apex". It may refer to:
*Apical ancestor, refers to the last common ancestor of an entire group, such as a species (biology) or a clan (anthropology)
*Apical (anatomy), an anatomical term of location for features loc ...
atom, as the carbon atom at the top of the pyramid. This approximation will provide insight into the intrinsic stability of the structures.
Apical carbon atom
Theapical
Apical means "pertaining to an apex". It may refer to:
*Apical ancestor, refers to the last common ancestor of an entire group, such as a species (biology) or a clan (anthropology)
*Apical (anatomy), an anatomical term of location for features loc ...
carbon atom is connected to only one other substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
, so an sp- hybridisation is to be expected. The substituent will be oriented upward. Towards the basic polygon, three orbitals are available:
* The second sp-orbital. This orbital is relatively low in energy due to the contribution of the s-orbital. With respect to the nodal planes in the remaining p-orbitals the symmetry of this orbital can be written as SxSy,S indicating the orbital is symmetric with respect to the plane indicated by the subscript. An A describes an anti-symmetry with respect to the by the subscript indicated plane. symmetric with respect to both planes. The orbital has a rather low energy, in terms of the Hückel method
The Hückel method or Hückel molecular orbital theory, proposed by Erich Hückel in 1930, is a simple LCAO MO Method, method for calculating molecular orbitals as linear combinations of atomic orbitals. The theory predicts the molecular orbitals f ...
its value is not easy to estimate, although it will be lower than α, since the orbital will have considerable s-character.
* Two p-orbitals. These orbitals have a higher energy content then the sp-orbital. In terms of the Huckel method, the energy will be α. In symmetry terms, these orbitals are orthogonal
In mathematics, orthogonality (mathematics), orthogonality is the generalization of the geometric notion of ''perpendicularity''. Although many authors use the two terms ''perpendicular'' and ''orthogonal'' interchangeably, the term ''perpendic ...
, described as AxSy and SxAy
Base of the pyramid
The approximation for the base of the pyramid is a closed ring of carbon atoms, all of them sp2 hybridised. The exact results depend on the ring size; overall conclusions can be formulated as: * The lowest molecular orbital has, watched from the apex of the pyramid, no nodal planes. Symmetry will be SxSy. In the Hückel method, its energy is * The next level of energy is occupied by two degenerated orbitals. In symmetry terms, they are written as SxAy and AxSy. The energy depends on the size of the ring: * Depending on the size of the base, there will be other MOs, but they are irrelevant to the present discussion.Interaction between apex and base
To obtain bonding interactions between atoms or parts of molecules, two conditions should be met: * The orbitals to combine should have the same symmetry. * A smaller difference in energy between the combining orbitals will produce a greater stabilizing effect. The orbitals at the apical carbon and the basic polygon are able to combine with respect to their symmetries. The result will be a more stable configuration for the pyramids. In figure 2, the symmetry aspects are depicted. * The apical sp orbital combines with the lowest MO of the basic ring to a low bonding and a high anti-bonding orbital. * The two apical p orbitals combine with the second lowest energy levels in the basic ring. Two bonding and two anti-bonding orbitals result. Figure 3 is a graphical representation of the results. Filling the atomic and molecular orbitals in pyramidal structures of different base size leads to the next table. Only bonding orbitals are accounted for. In the case of the three-sided pyramid, clearly no ion results; a known neutral species arises:tetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized . However, a number of derivatives have been prepa ...
. To this molecule this way of description is an alternative quantum mechanical description.
The other pyramidal structures will be charged in relation with their base size.
Examples
Monocation
: Figure 4: A number of derivatives of tricyclo ,1,0,02,4entane (TCP) leading to the same pyramidal cation. The carbon atom carrying theleaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
becomes basic, while carbon at the ''anti'' position becomes apical.The group "R" is either 1H or 2H ( D):
3-hydroxy-1,4-dimethyl-TCP 3HO-1,5-diMe-tricyclo(2,1,0,02,5)pentaan.jpg, 4b:
3-hydroxy-1,5-dimethyl-TCP 3MeO-3,5-diMe-tricyclo(2,1,0,02,5)pentaan.jpg, 4c:
3-methoxy-3,5-dimethyl-TCP Four sided Pyramidal 1,5-DiMe-3-R.jpg, 4d:
resulting carbocation
superacid
In chemistry, a superacid (according to the original definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superacid i ...
(a mixture of SO2ClF and FSO3H). Based on both the 13C as well as the 1H-NMR-spectrum the evidence is clear: in each case the same intermediary is formed. Also, when the super acidic medium is destroyed, with either methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
or benzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
, the same product is formed. (see: Reaction... ''below'').
* Assignment in the hydrogen spectrum is partly on intensity (hydrogens at the basic ring) partly on the common experience hydrogen's at the outer side of a circular conjugated system have signals at higher ppm relative to TMS, while those positioned over the ring will have lower, even negative, signals relative to TMS.
* Assignment in 13C-NMR follow the same considerations as for 1H. Though in carbon NMR intensity is a bad guide to the number of atoms, in the basic ring the unsubstituted carbons are similar enough to use intensity as indication for their number. A powerful tool too is the multiplicity of the carbon signal due to coupling with the to carbon bonded hydrogens.
* Masamune himself does not state anything about the nature of the intermediate ion. Nevertheless, based on rules formulated by Olah, he is able to rule out localized cations (like 1-butyl) or delocalized ones (like allyl). For those ions signals around 200 ppm are expected.
Reaction with methanol and benzoic acid
: Figure 5: De reaction products of the dimethyl pyramidal cation with methanol and benzoic acid.The "R"-group is either1H or 2H ( D):
Pyramidal cation 3BzO-1,4-diMe-tricyclo(2,1,0,02,5)pentaan.jpg, 5b
Reaction product with benzoic acid
in dipolar aprotic solvent 3MeO-3,5-diMe-tricyclo(2,1,0,02,5)pentaan.jpg, 5c
reaction product with methanol
in methanol
electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
substituent (2.5 versus 2.1 on the Pauling scale) will concentrate charge on the skeletal carbon. This charge concentration has several effects:
* The reaction with benzoate is governed by π - π interactions. The degeneration in the basic MO-system will be lost because of the presence of a substituting methyl group. As the apical side is inaccessible, benzoate will approach from the bottom side of the pyramid. The interaction between the two π-systems, both disturbed at one point, will force a specific orientation. The orientation in which the interaction between positive charge generated by the methyl group on the pyramid and the charge adjacent to the carboxyl group will direct the system to a reaction of the carboxyl group with carbon 2 or 4 of the pyramid base. When reaction with benzoate takes place at carbon 2 bridges will form between the apical carbon and atoms 1 and 3. A bond too will form between ''anti''-carbon 4 and the apex. Reaction at carbon 4 will have a same effect, although the resulting molecule has a mirror relation with respect to the molecule that results from reaction at position 2.
* The reaction with methanol is charge driven. In de basic system an identifiable center of positive charge is present at the carbon carrying the methyl group. Methanol with its hard base
HSAB is an acronym for "hard and soft (Lewis) acids and bases". HSAB is widely used in chemistry for explaining the stability of compounds, reaction mechanisms and pathways. It assigns the terms 'hard' or 'soft', and 'acid' or 'base' to chemical ...
in oxygen, will react at the center of positivity. The methoxy group appears at carbon 1, forcing bridges to form between carbon 2 and 4 to the apex, as well as between the now ''anti''-carbon 3.
Bishomomonocarbocation
In chemistry, the prefix "homo-" denotes ahomolog
In biology, homology is similarity in anatomical structures or genes between organisms of different taxa due to shared ancestry, ''regardless'' of current functional differences. Evolutionary biology explains homologous structures as retained her ...
, a likewise compound containing one, or as in this case two, extra CH2-groups. The common aspect of the bishomo ions is the possession of a 1,4-cyclohexadiene
1,4-Cyclohexadiene is an organic compound with the formula C6H8. It is a colourless, flammable liquid that is of academic interest as a prototype of a large class of related compounds called terpenoids, an example being γ-terpinene. An isomer of ...
ring instead of a cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is ...
one.
norbornadiene
Norbornadiene is an organic compound and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distin ...
, creates an even more stable structure.
Dication
According to the results presented in Table 1, a five-sided pyramidal carbocation will be divalent. This is confirmed by theoretical and practical work by Hogeveen. In contrast to the monocation, which is described with several patterns of substitution, the dication is mainly studied by its hexamethyl derivative. The synthesis starts athexamethyl Dewar benzene
Hexamethyl Dewar benzene is a derivative of Dewar benzene with application in organometallic chemistry. It consists of the Dewar benzene core, with a methyl group substituent on each of its six carbon positions.
Synthesis
Hexamethyl Dewar benze ...
(compound I in table 4) reacting with Cl2 into 5,6-dichloro-1,2,3,4,5,6-hexamethylbicyclo .1.1ex-2-ene (compound II in table 4). Dissolution of this compound in fluorosulfonic acid
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula . It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, , substitu ...
gives rise to the dication (structure III in table 4).
The presence of a pyramidal ion in the solution of fluorosulfonic acid is evidenced by the 1H- and 13C-NMR-spectrum (Table 5).
The assignment of the signals is based on their intensities and multiplicities. The assignment of the pyramidal structure is based on the observed simplicity of the spectra: five equal C-CH3 groups combined with one outstanding C-CH3 group. The only way to construct a molecular entity from this data is a five-sided pyramid. Rapid equilibriums between degenerated classical or non-classical carbocations are discarded as the position of the signals does not match the expected values for those kind of structures.
The crystal structure of 6(CH3)6sup>2+ (SbF6−)2 • HSO3F was obtained in 2017. Although the apical carbon atom is hexacoordinated, the rule of the tetravalency of carbon is still fulfilled. While the C-CH3 bond length of 1.479(3) Å is typical for a C-C single bond, the other five very long C-C distances of 1.694(2)-1.715(3) Å indicate a bond order of <1.
Reactions of the dication
The reactions of the dication fall apart into three groups: * Thermal reactions The hexamethyl substituted dication is a stable structure up to . Above this temperature reaction occurs: ahydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
-ion is taken up, followed by an irreversible rearrangement to an arenium ion
An arenium ion in organic chemistry is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution.
For historic reasons this complex is also called a Wheland intermediate, after American chemist George ...
which is stable in the fluorosulfonic acid medium (see: Figure 6, upper reaction).
* Charged nucleophiles (hydride, methoxide, hydroxide) react reversible, leading to, independent of the nucleofile at hand, identical 2,4-substituted tricyclo .1.0.03,6exane derivatis, e.g.: with methoxide: 2,4-dimethoxy-tricyclo .1.0.03,6exane is formed (see: Figure 6, middle reaction path).
* Uncharged nucleofiles (amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s like triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
) act as a base, reversible extracting two hydrogens from the ion, in effect producing a dimethylene derivative of benzvalene
Benzvalene is an organic compound and one of several isomers of benzene. It was first synthesized in 1967 by K. E. Wilzbach et al. via photolysis of benzene and the synthesis was later improved by Thomas J. Katz et al.
The 1971 synthesis consist ...
(see: Figure 6, lower reaction).
Other substitution patterns at the dications
The product of the reaction of the dication withtriethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
offers a pathway to other substitution patterns then hexamethyl. One or both double bonds are oxidized to a keton. The keton then is reacted with an organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and ...
producing an alkylated hydroxide. The compounds formed in this way possess one or two other alkyl groups, depending on the number of oxidized double bonds. When the alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s are dissolved in fluorosulfonic acid
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula . It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, , substitu ...
, they again give rise to new pyramidal dications. Both non-methyl groups occupy basal positions. Each other position at the pyramidal skeleton still carries a methyl group. Table 6 summarizes these findings.
Up to this point the substitution pattern of the divalent pyramidal ion is of minor importance to its behavior. A clear difference arises when the thermal stability if the ions of type V (Table 6) is studied: {{nowrap, 1=at {{cvt, -40, °C the apical ethyl substituted ion is stable for 48 hours, whereas no trace of the apical ''iso''-propyl ion is detectable anymore.
Tervalent and higher ions
At the time of the literature survey (end of 1978), there were no reports on tervalent or higher pyramidal cations.Notes and references