Protein Tandem Repeats on:
[Wikipedia]
[Google]
[Amazon]
 An array of protein tandem repeats is defined as several (at least two) adjacent copies having the same or similar
An array of protein tandem repeats is defined as several (at least two) adjacent copies having the same or similar 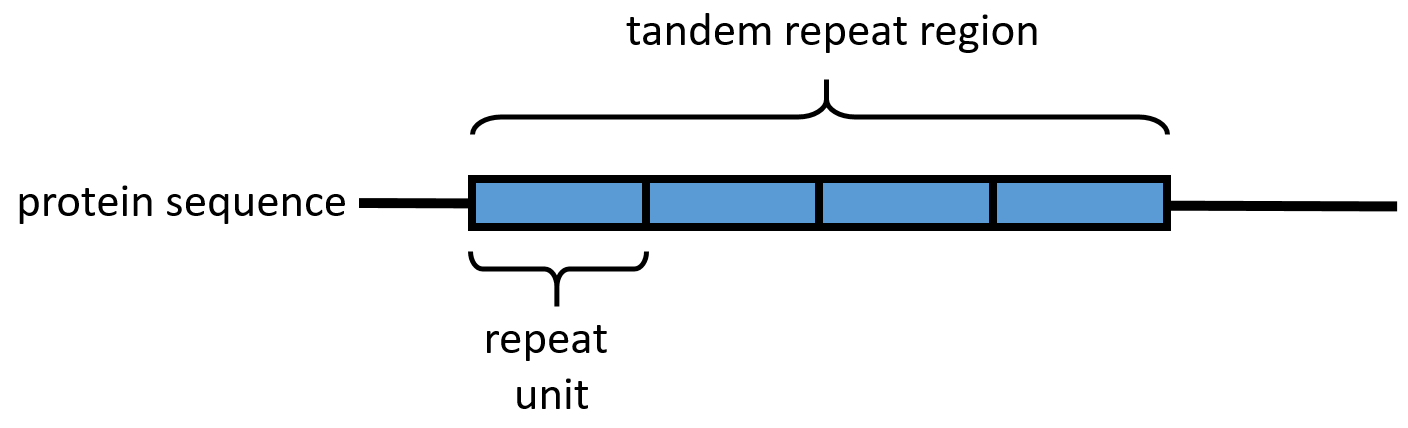
 In
In
RepeatsDB: a database of annotated tandem repeat protein structures
{{Protein tandem repeats Protein tandem repeats Protein domains
 An array of protein tandem repeats is defined as several (at least two) adjacent copies having the same or similar
An array of protein tandem repeats is defined as several (at least two) adjacent copies having the same or similar sequence motif
In biology, a sequence motif is a nucleotide or amino-acid sequence pattern that is widespread and usually assumed to be related to biological function of the macromolecule. For example, an ''N''-glycosylation site motif can be defined as ''A ...
s. These periodic sequences are generated by internal duplications in both coding and non-coding genomic sequences. Repetitive units of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
tandem repeats are considerably diverse, ranging from the repetition of a single amino acid to domains of 100 or more residues.

"Repeats" in proteins
 In
In protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s, a "repeat" is any sequence block that returns more than one time in the sequence
In mathematics, a sequence is an enumerated collection of objects in which repetitions are allowed and order matters. Like a set, it contains members (also called ''elements'', or ''terms''). The number of elements (possibly infinite) is cal ...
, either in an identical or a highly similar form. The degree of similarity can be highly variable, with some repeats maintaining only a few conserved amino acid positions and a characteristic length. Highly degenerate repeats can be very difficult to detect from sequence alone. Structural similarity can help to identify repetitive patterns in sequence.
Structure
Repetitiveness does not in itself indicate anything about the structure of the protein. As a "rule of thumb", short repetitive sequences (e.g. those below the length of 10 amino acids) may be intrinsically disordered, and not part of any foldedprotein domain
In molecular biology, a protein domain is a region of a protein's Peptide, polypeptide chain that is self-stabilizing and that Protein folding, folds independently from the rest. Each domain forms a compact folded Protein tertiary structure, thre ...
s. Repeats that are at least 30 to 40 amino acids long are far more likely to be folded as part of a domain. Such long repeats are frequently indicative of the presence of a solenoid domain in the protein.
Approximately half of the tandem repeat regions have intrinsically disordered conformation being naturally unfolded. Examples of disordered repetitive sequences include the 7-mer peptide repeats found in the RPB1 subunit of RNA polymerase II
RNA polymerase II (RNAP II and Pol II) is a Protein complex, multiprotein complex that Transcription (biology), transcribes DNA into precursors of messenger RNA (mRNA) and most small nuclear RNA (snRNA) and microRNA. It is one of the three RNA pol ...
, or the tandem beta-catenin
Catenin beta-1, also known as β-catenin (''beta''-catenin), is a protein that in humans is encoded by the ''CTNNB1'' gene.
β-Catenin is a dual function protein, involved in regulation and coordination of cell–cell adhesion and gene transcrip ...
or axin binding linear motifs in APC (adenomatous polyposis coli). The other half of the regions with the stable 3D structure has a plethora of shapes and functions. Examples of short repeats exhibiting ordered structures include the three-residue collagen repeat or the five-residue pentapeptide repeat that forms a beta helix structure.
Classification
Depending on the length of the repetitive units, their protein structures can be subdivided into five classes: # crystalline aggregates formed by regions with 1 or 2 residue long repeats, archetypical low complexity regions # fibrous structures stabilized by inter-chain interactions with 3-7 residue repeats # elongated structures with repeats of 5–40 residues dominated by solenoid proteins # closed (not elongated) structures with repeats of 30-60 residues as toroid repeats # beads on a string structures with typical size of repeats over 50 residues, which are already large enough to fold independently into stable domains.Function
Some well-known examples of proteins with tandem repeats arecollagen
Collagen () is the main structural protein in the extracellular matrix of the connective tissues of many animals. It is the most abundant protein in mammals, making up 25% to 35% of protein content. Amino acids are bound together to form a trip ...
, which plays a key role in the arrangement of the extracellular matrix; alpha-helical coiled coils having structural and oligomerization functions; leucine-rich repeat
A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe tertiary structure, fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These Pr ...
proteins, which specifically bind some globular proteins by their concave surfaces; and zinc-finger proteins, which regulate the expression of genes by binding DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
.
Tandem repeat proteins frequently function as protein-protein interaction modules. The WD40 repeat
The WD40 repeat (also known as the WD or beta-transducin repeat) is a short structural motif of approximately 40 amino acids, often terminating in a tryptophan-aspartic acid (W-D) dipeptide. Tandem copies of these repeats typically fold togethe ...
is a prime example of this function.
Distribution in proteomes
Tandem repeats are ubiquitous inproteome
A proteome is the entire set of proteins that is, or can be, expressed by a genome, cell, tissue, or organism at a certain time. It is the set of expressed proteins in a given type of cell or organism, at a given time, under defined conditions. P ...
s and occur in at least 14% of all proteins. For example, they are present in almost every third human protein and even in every second protein from Plasmodium falciparum
''Plasmodium falciparum'' is a Unicellular organism, unicellular protozoan parasite of humans and is the deadliest species of ''Plasmodium'' that causes malaria in humans. The parasite is transmitted through the bite of a female ''Anopheles'' mos ...
or Dictyostelium discoideum
''Dictyostelium discoideum'' is a species of soil-dwelling Amoeboid, amoeba belonging to the phylum Amoebozoa, infraphylum Mycetozoa. Commonly referred to as slime mold, ''D. discoideum'' is a eukaryote that transitions from a collection of unic ...
. Tandem repeats with short repetitive units (especially homorepeats) are more frequent than others.
Annotation methods
Protein tandem repeats can be either detected from sequence or annotated from structure. Specialized methods were built for the identification of repeat proteins. Sequence-based strategies, based on homology search or domain assignment, mostly underestimate TRs due to the presence of highly degenerate repeat units. A recent study to understand and improve Pfam coverage of the human proteome showed that five of the ten largest sequence clusters not annotated with Pfam are repeat regions. Alternatively, methods requiring no prior knowledge for the detection of repeated substrings can be based on self-comparison, clustering or hidden Markov models. Some others rely on complexity measurements or take advantage of meta searches to combine outputs from different sources. Structure-based methods instead take advantage of the modularity of available PDB structures to recognize repetitive elements.References
External links
RepeatsDB: a database of annotated tandem repeat protein structures
{{Protein tandem repeats Protein tandem repeats Protein domains