Precoccinelline on:
[Wikipedia]
[Google]
[Amazon]
Precoccinelline is an

alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large varie ...
produced by the ''Coccinella septempunctata
''Coccinella septempunctata'', the common ladybug, the seven-spot ladybird (or, in North America, seven-spotted ladybug or "C-7"), is a carnivorous beetle native to Europe, Africa and Eastern Asia. It has been introduced to North America and ca ...
'', also known as the seven-spot ladybird. The alkaloid is released from the joints in ''C. septempunctata'' legs when it is provoked to deter predators such as ants or birds. It binds to both insect and mammalian nicotinic acetylcholine receptors, giving it use as an insecticide or as a therapy to treat drug dependence.
Biosynthesis
It was determined that precoccinelline is produced de novo in the ''C. septempunctata'' as their diet, which is composed ofaphids
Aphids are small sap-sucking insects in the Taxonomic rank, family Aphididae. Common names include greenfly and blackfly, although individuals within a species can vary widely in color. The group includes the fluffy white Eriosomatinae, woolly ...
, does not contain this alkaloid. While the exact biosynthesis has not been fleshed out, precoccinelline is thought to be of polyketide
In organic chemistry, polyketides are a class of natural products derived from a Precursor (chemistry), precursor molecule consisting of a Polymer backbone, chain of alternating ketone (, or Carbonyl reduction, its reduced forms) and Methylene gro ...
origin. The hypothesized biosynthesis involves condensation of six acetates and methyl addition.
:
Chemical synthesis

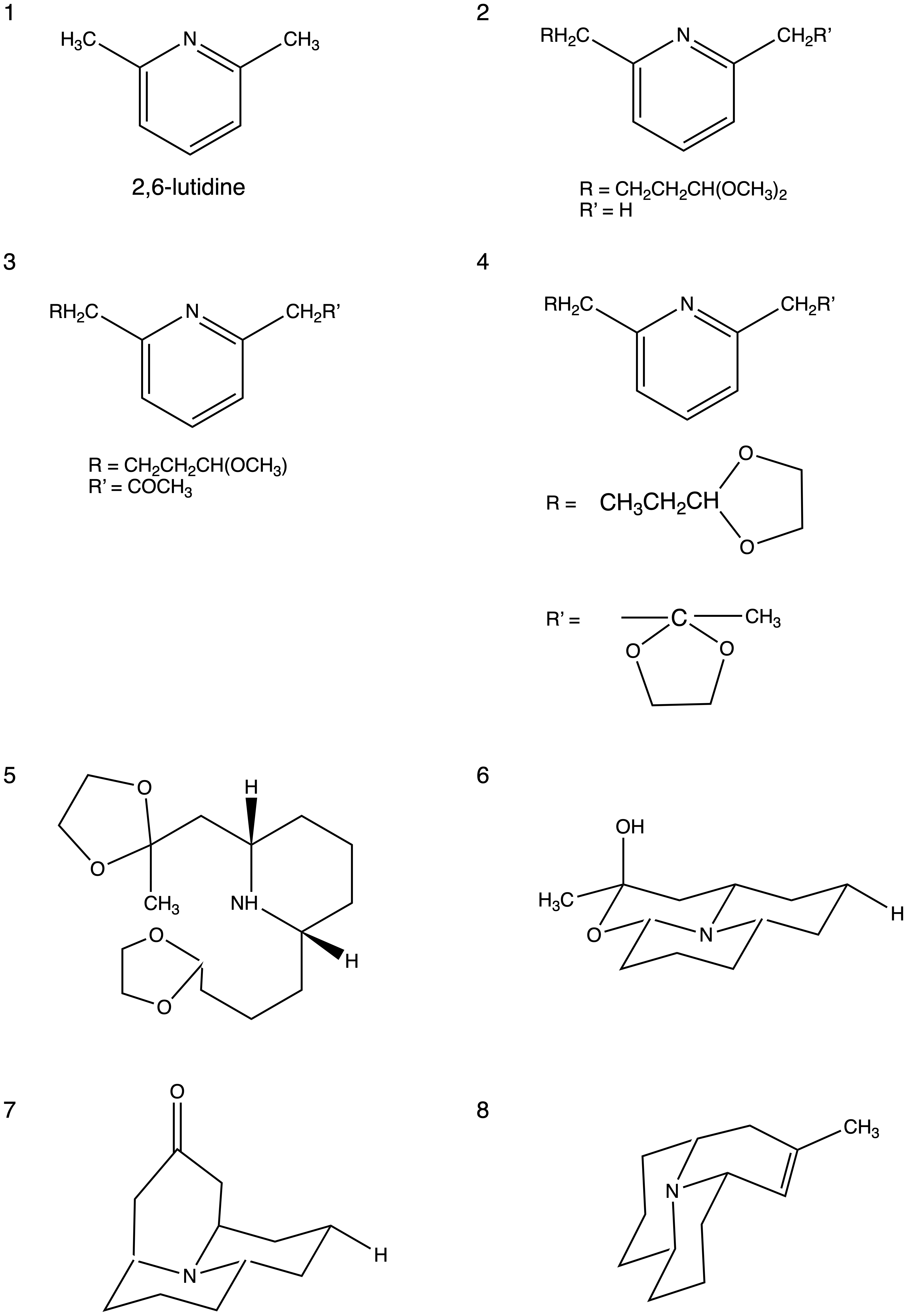
2,6-lutidine
2,6-Lutidine is a natural heterocyclic aromatic organic compound with the formula (CH3)2C5H3N. It is one of several dimethyl-substituted derivative of pyridine, all of which are referred to as Lutidine, lutidines. It is a colorless liquid with mi ...
(1) is used as starting material, and its treatment with β-bromo-propionaldehyde dimethyl acetal in ether produces a monolithium derivative. In the presence of 2,6-lutidine
2,6-Lutidine is a natural heterocyclic aromatic organic compound with the formula (CH3)2C5H3N. It is one of several dimethyl-substituted derivative of pyridine, all of which are referred to as Lutidine, lutidines. It is a colorless liquid with mi ...
in excess, the monolithium derivative becomes an acetal (2). Treatment of the acetal with phenyl-lithium and then adding an ethereal solution of acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
produces the crude ketone (3), followed by immediate transformation into the diacetal (4). Using sodium-isoamyl alcohol, the diacetal can be reduced to give the ''cis'' piperidine (5). The ''trans''-isomer of the ''cis'' piperidine can be isolated and hydrolyzed using aqueous hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
to yield the ketol (6). The ketol can then be cyclized with the use of acetic acid and pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most ...
in refluxing tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
which gives a mixture of ketones. Separation of structure 7 from the mixture and then treatment with methyllithium
Methyllithium is the simplest organolithium reagent, with the empirical formula LiCH3. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used i ...
in ether to produce a carbinol (8). Dehydrating the carbinol with thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately Volatility (chemistry), volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a Halogenation, chlorinating reagen ...
in methylene chloride gives olefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
which can be hydrogenated to produce precoccinelline.
Mechanism of action
Precoccinelline is an alkaloid which acts as an inhibitor ofnicotinic acetylcholine receptors
Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral ner ...
(nAChRs). It binds to an allosteric site
In the fields of biochemistry and pharmacology an allosteric regulator (or allosteric modulator) is a substance that binds to a site on an enzyme or receptor distinct from the active site, resulting in a conformational change that alters the p ...
on nAChRs― a site separate from the ACh recognition site. Of the secreted ladybird alkaloids, precoccinelline was the most potent inhibitor, acting via a non-competitive
Non-competitive inhibition is a type of enzyme inhibition where the inhibitor reduces the activity of the enzyme and binds equally well to the enzyme regardless of whether it has already bound the substrate. This is unlike competitive inhibition, ...
mechanism.
Targeting nAChRs has several implications including developing insecticides and modulating drug dependence relating to the reward pathway
The mesolimbic pathway, sometimes referred to as the reward pathway, is a dopaminergic pathway in the brain. The pathway connects the ventral tegmental area in the midbrain to the ventral striatum of the basal ganglia in the forebrain. The ventral ...
in the brain.
References
{{Reflist Heterocyclic compounds with 3 rings Nitrogen heterocycles Alkaloids Acetylcholinesterase inhibitors