Practical salinity unit on:
[Wikipedia]
[Google]
[Amazon]
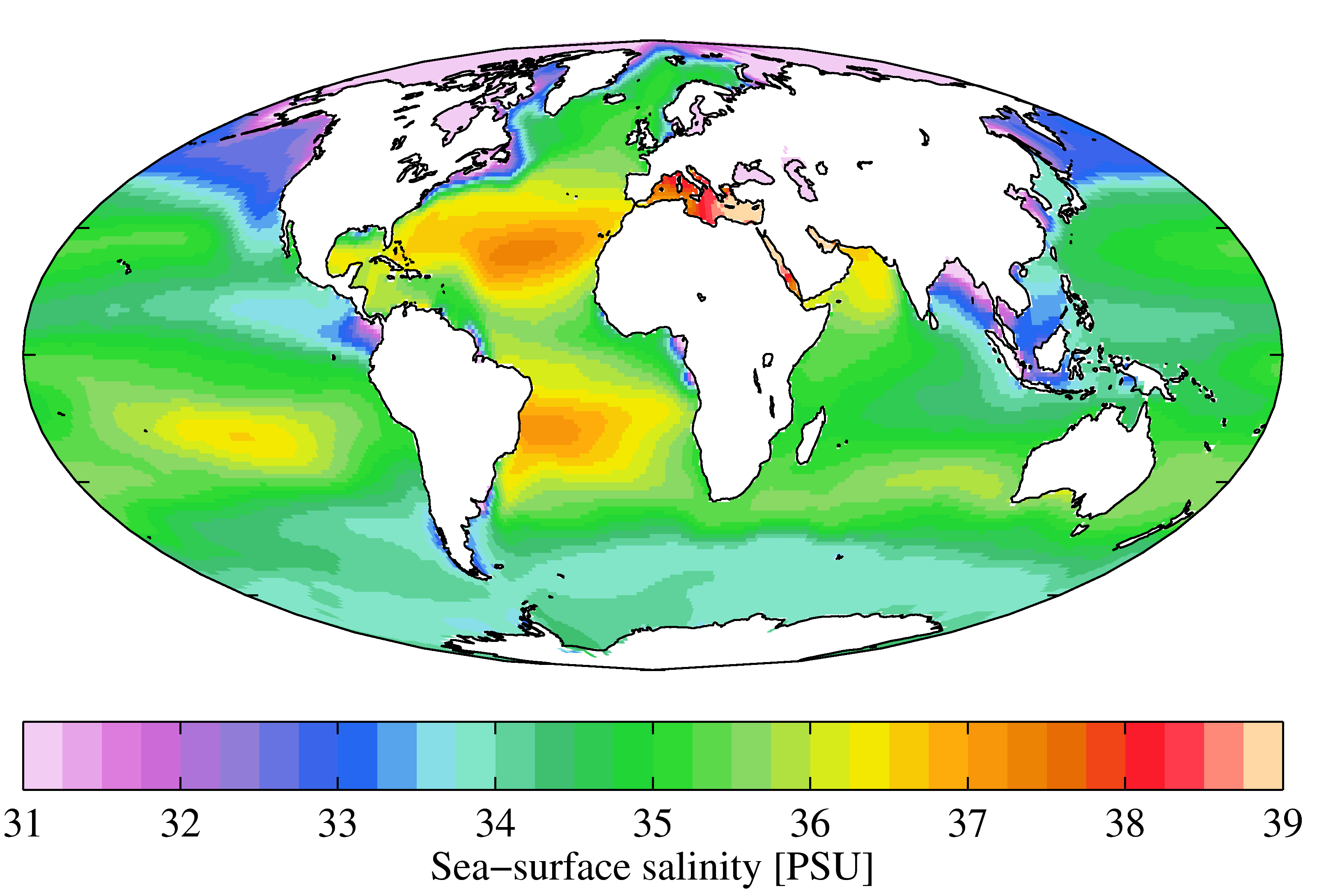 Salinity () is the saltiness or amount of
Salinity () is the saltiness or amount of
Background papers and supporting data on the Practical Salinity Scale 1978
''Tech. Pap. Mar. Sci.'', 37 Salinities measured using PSS-78 do not have units. The suffix psu or PSU (denoting ''practical salinity unit'') is sometimes added to PSS-78 measurement values. The addition of PSU as a unit after the value is "formally incorrect and strongly discouraged". In 2010 a new standard for the properties of seawater called the ''thermodynamic equation of seawater 2010'' (
 Salinity () is the saltiness or amount of
Salinity () is the saltiness or amount of salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
dissolved in a body of water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
, called saline water
Saline water (more commonly known as salt water) is water that contains a high concentration of dissolved salts (mainly sodium chloride). On the United States Geological Survey (USGS) salinity scale, saline water is saltier than brackish wat ...
(see also soil salinity
Soil salinity is the salt (chemistry), salt content in the soil; the process of increasing the salt content is known as salinization (also called salination in American and British English spelling differences, American English). Salts occur nat ...
). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to ‰).
Salinity is an important factor in determining many aspects of the chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
of natural waters and of biological
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, origin, evolution, and distribution of ...
processes within it, and is a thermodynamic state variable that, along with temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
and pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
, governs physical characteristics like the density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be u ...
and heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity is a ...
of the water.
A contour line
A contour line (also isoline, isopleth, isoquant or isarithm) of a Function of several real variables, function of two variables is a curve along which the function has a constant value, so that the curve joins points of equal value. It is a ...
of constant salinity is called an ''isohaline'', or sometimes ''isohale''.
Definitions
Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds likesodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
, magnesium sulfate
Magnesium sulfate or magnesium sulphate is a chemical compound, a salt with the formula , consisting of magnesium cations (20.19% by mass) and sulfate anions . It is a white crystalline solid, soluble in water but not in ethanol.
Magnesi ...
, potassium nitrate
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula . It is a potassium salt of nitric acid. This salt consists of potassium cations and nitrate anions , and is therefore an alkali metal nit ...
, and sodium bicarbonate
Sodium bicarbonate ( IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda (or simply “bicarb” especially in the UK) is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cat ...
which dissolve into ions. The concentration of dissolved chloride ions is sometimes referred to as chlorinity. Operationally, dissolved matter is defined as that which can pass through a very fine filter (historically a filter with a pore size of 0.45 μm, but later usually 0.2 μm). Salinity can be expressed in the form of a mass fraction, i.e. the mass of the dissolved material in a unit mass of solution.
Seawater typically has a mass salinity of around 35 g/kg, although lower values are typical near coasts where rivers enter the ocean. Rivers and lakes can have a wide range of salinities, from less than 0.01 g/kg to a few g/kg, although there are many places where higher salinities are found. The Dead Sea
The Dead Sea (; or ; ), also known by #Names, other names, is a landlocked salt lake bordered by Jordan to the east, the Israeli-occupied West Bank to the west and Israel to the southwest. It lies in the endorheic basin of the Jordan Rift Valle ...
has a salinity of more than 200 g/kg. Precipitation typically has a TDS of 20 mg/kg or less.
Whatever pore size is used in the definition, the resulting salinity value of a given sample of natural water will not vary by more than a few percent
In mathematics, a percentage () is a number or ratio expressed as a fraction of 100. It is often denoted using the ''percent sign'' (%), although the abbreviations ''pct.'', ''pct'', and sometimes ''pc'' are also used. A percentage is a dime ...
(%). Physical oceanographers working in the abyssal ocean, however, are often concerned with precision and intercomparability of measurements by different researchers, at different times, to almost five significant digits
Significant figures, also referred to as significant digits, are specific digits within a number that is written in positional notation that carry both reliability and necessity in conveying a particular quantity. When presenting the outcom ...
. A bottled seawater product known as IAPSO Standard Seawater is used by oceanographers to standardize their measurements with enough precision to meet this requirement.
Composition
Measurement and definition difficulties arise because natural waters contain a complex mixture of many different elements from different sources (not all from dissolved salts) in different molecular forms. The chemical properties of some of these forms depend on temperature and pressure. Many of these forms are difficult to measure with high accuracy, and in any case complete chemical analysis is not practical when analyzing multiple samples. Different practical definitions of salinity result from different attempts to account for these problems, to different levels of precision, while still remaining reasonably easy to use. For practical reasons salinity is usually related to the sum of masses of a subset of these dissolved chemical constituents (so-called ''solution salinity''), rather than to the unknown mass of salts that gave rise to this composition (an exception is when artificial seawater is created). For many purposes this sum can be limited to a set of eight major ions in natural waters, although for seawater at highest precision an additional seven minor ions are also included. The major ions dominate the inorganic composition of most (but by no means all) natural waters. Exceptions include some pit lakes and waters from some hydrothermal springs. The concentrations of dissolved gases likeoxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
are not usually included in descriptions of salinity. However, carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
gas, which when dissolved is partially converted into carbonates
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group . ...
and bicarbonates
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemica ...
, is often included. Silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
in the form of silicic acid
In chemistry, a silicic acid () is any chemical compound containing the element silicon attached to oxide () and hydroxyl () groups, with the general formula or, equivalently, . Orthosilicic acid is a representative example. Silicic acids are ra ...
, which usually appears as a neutral molecule in the pH range of most natural waters, may also be included for some purposes (e.g., when salinity/density relationships are being investigated).
Seawater
The term ''salinity'' is, for oceanographers, usually associated with one of a set of specific measurement techniques. As the dominant techniques evolve, so do different descriptions of salinity. Salinities were largely measured usingtitration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of Quantitative research, quantitative Analytical chemistry, chemical analysis to determine the concentration of an identified analyte (a substance to be ...
-based techniques before the 1980s. Titration with silver nitrate
Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar causti ...
could be used to determine the concentration of halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
ions (mainly chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
and bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
) to give a chlorinity. The chlorinity was then multiplied by a factor to account for all other constituents. The resulting 'Knudsen salinities' are expressed in units of parts per thousand
In science and engineering, the parts-per notation is a set of pseudo-units to describe the small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction.
Since these fractions are quantity-per-quantity measures ...
(ppt or ‰).
The use of electrical conductivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity in ...
measurements to estimate the ionic content of seawater led to the development of the scale called the ''practical salinity scale 1978'' (PSS-78).Unesco (1981). The Practical Salinity Scale 1978 and the International Equation of State of Seawater 1980. ''Tech. Pap. Mar. Sci.'', 36Unesco (1981)Background papers and supporting data on the Practical Salinity Scale 1978
''Tech. Pap. Mar. Sci.'', 37 Salinities measured using PSS-78 do not have units. The suffix psu or PSU (denoting ''practical salinity unit'') is sometimes added to PSS-78 measurement values. The addition of PSU as a unit after the value is "formally incorrect and strongly discouraged". In 2010 a new standard for the properties of seawater called the ''thermodynamic equation of seawater 2010'' (
TEOS-10
TEOS-10 (Thermodynamic Equation of Seawater - 2010) is the international standard for the use and calculation of the thermodynamic properties of seawater, humid air and ice. It supersedes the former standard EOS-80 (Equation of State of Seawater 1 ...
) was introduced, advocating absolute salinity as a replacement for practical salinity, and conservative temperature as a replacement for potential temperature. This standard includes a new scale called the ''reference composition salinity scale''. Absolute salinities on this scale are expressed as a mass fraction, in grams per kilogram of solution. Salinities on this scale are determined by combining electrical conductivity measurements with other information that can account for regional changes in the composition of seawater. They can also be determined by making direct density measurements.
A sample of seawater from most locations with a chlorinity of 19.37 ppt will have a Knudsen salinity of 35.00 ppt, a PSS-78 practical salinity of about 35.0, and a TEOS-10 absolute salinity of about 35.2 g/kg. The electrical conductivity of this water at a temperature of 15 °C is 42.9 mS/cm.
On the global scale, it is extremely likely that human-caused climate change has contributed to observed surface and subsurface salinity changes since the 1950s, and projections of surface salinity changes throughout the 21st century indicate that fresh ocean regions will continue to get fresher and salty regions will continue to get saltier.
Salinity is serving as a tracer of different masses. Surface water is pulled in to replace the sinking water, which in turn eventually becomes cold and salty enough to sink. Salinity distribution contributes to shape the oceanic circulation.
Lakes and rivers
Limnologist
Limnology ( ; ) is the study of inland aquatic ecosystems.
It includes aspects of the biological, chemical, physical, and geological characteristics of fresh and saline, natural and man-made bodies of water. This includes the study of lakes, ...
s and chemists often define salinity in terms of mass of salt per unit volume, expressed in units of mg/L or g/L. It is implied, although often not stated, that this value applies accurately only at some reference temperature because solution volume varies with temperature. Values presented in this way are typically accurate to the order of 1%. Limnologists also use electrical conductivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity in ...
, or "reference conductivity", as a proxy for salinity. This measurement may be corrected for temperature effects, and is usually expressed in units of μS/cm.
A river or lake water with a salinity of around 70 mg/L will typically have a specific conductivity at 25 °C of between 80 and 130 μS/cm. The actual ratio depends on the ions present. The actual conductivity usually changes by about 2% per degree Celsius, so the measured conductivity at 5 °C might only be in the range of 50–80 μS/cm.
Direct density measurements are also used to estimate salinities, particularly in highly saline lakes. Sometimes density at a specific temperature is used as a proxy for salinity. At other times an empirical salinity/density relationship developed for a particular body of water is used to estimate the salinity of samples from a measured density.
Classification of water bodies based upon salinity
Marine waters are those of the ocean, another term for which is ''euhaline seas''. The salinity of euhaline seas is 30 to 35 ‰. ''Brackish seas'' or waters have salinity in the range of 0.5 to 29 ‰ and ''metahaline seas'' from 36 to 40 ‰. These waters are all regarded as ''thalassic'' because their salinity is derived from the ocean and defined as ''homoiohaline'' if salinity does not vary much over time (essentially constant). The table on the right, modified from Por (1972), follows the "Venice system" (1959). In contrast to homoiohaline environments are certain ''poikilohaline'' environments (which may also be ''thalassic'') in which the salinity variation is biologically significant. ''Poikilohaline'' water salinities may range anywhere from 0.5 to greater than 300 ‰. The important characteristic is that these waters tend to vary in salinity over some biologically meaningful range seasonally or on some other roughly comparable time scale. Put simply, these are bodies of water with quite variable salinity. Highly saline water, from which salts crystallize (or are about to), is referred to asbrine
Brine (or briny water) is a high-concentration solution of salt (typically sodium chloride or calcium chloride) in water. In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawat ...
.
Environmental considerations
Salinity is an ecological factor of considerable importance, influencing the types of organisms that live in a body of water. As well, salinity influences the kinds ofplant
Plants are the eukaryotes that form the Kingdom (biology), kingdom Plantae; they are predominantly Photosynthesis, photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with c ...
s that will grow either in a water body, or on land fed by a water (or by a groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit ...
). A plant adapted to saline conditions is called a halophyte
A halophyte is a salt-tolerant plant that grows in soil or waters of high salinity, coming into contact with saline water through its roots or by salt spray, such as in saline semi-deserts, mangrove swamps, marshes and sloughs, and seashores. ...
. A halophyte which is tolerant to residual sodium carbonate salinity are called glasswort or saltwort or barilla
''Barilla'' refers to several species of salt-tolerant (halophyte) plants that, until the 19th century, were the primary source of soda ash and hence of sodium carbonate. The word "barilla" was also used directly to refer to the soda ash obtain ...
plants. Organisms (mostly bacteria) that can live in very salty conditions are classified as extremophile
An extremophile () is an organism that is able to live (or in some cases thrive) in extreme environments, i.e., environments with conditions approaching or stretching the limits of what known life can adapt to, such as extreme temperature, press ...
s, or halophile
A halophile (from the Greek word for 'salt-loving') is an extremophile that thrives in high salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more ...
s specifically. An organism that can withstand a wide range of salinities is euryhaline
Euryhaline organisms are able to adapt to a wide range of salinities. An example of a euryhaline fish is the short-finned molly, '' Poecilia sphenops'', which can live in fresh water, brackish water, or salt water.
The green crab ('' Carcinus m ...
.
Salts are expensive to remove from water, and salt content is an important factor in water use, factoring into potability
Drinking water or potable water is water that is safe for ingestion, either when drinking, drunk directly in liquid form or consumed indirectly through food preparation. It is often (but not always) supplied through taps, in which case it is al ...
and suitability for irrigation
Irrigation (also referred to as watering of plants) is the practice of applying controlled amounts of water to land to help grow crops, landscape plants, and lawns. Irrigation has been a key aspect of agriculture for over 5,000 years and has bee ...
. Increases in salinity have been observed in lakes and rivers in the United States, due to common road salt
Road salt (also known as de-icing salt, rock salt, or snow salt) is a salt used mainly as an anti-slip agent in winter road conditions, but also to prevent dust and snow build-up on roads. Various kinds of salts are used as road salt, but calciu ...
and other salt de-icers in runoff.
The degree of salinity in oceans is a driver of the world's ocean circulation, where density changes due to both salinity changes and temperature changes at the surface of the ocean produce changes in buoyancy, which cause the sinking and rising of water masses. Changes in the salinity of the oceans are thought to contribute to global changes in carbon dioxide as more saline waters are less soluble to carbon dioxide. In addition, during glacial periods, the hydrography
Hydrography is the branch of applied sciences which deals with the measurement and description of the physical features of oceans, seas, coastal areas, lakes and rivers, as well as with the prediction of their change over time, for the primary ...
is such that a possible cause of reduced circulation is the production of stratified oceans. In such cases, it is more difficult to subduct water through the thermohaline circulation.
Not only is salinity a driver of ocean circulation, but changes in ocean circulation also affect salinity, particularly in the subpolar North Atlantic where from 1990 to 2010 increased contributions of Greenland meltwater were counteracted by increased northward transport of salty Atlantic waters. However, North Atlantic waters have become fresher since the mid-2010s due to increased Greenland meltwater flux.
See also
*Desalination for economic purposes **Desalination
Desalination is a process that removes mineral components from saline water. More generally, desalination is the removal of salts and minerals from a substance. One example is Soil salinity control, soil desalination. This is important for agric ...
of water
**Desalination of soil: soil salinity control
Soil salinity control refers to controlling the process and progress of soil salinity to prevent soil degradation by salination and land reclamation, reclamation of already salty (saline) soils. Soil reclamation is also known as soil improvement ...
** Sodium adsorption ratio
* Measuring salinity
**Salinometer
A salinometer is a device designed to measure the salinity, or dissolved salt content, of a solution.
Since the salinity affects both the electrical conductivity and the specific gravity of a solution, a salinometer often consist of an ec mete ...
* Salinity by biologic context
** In organisms generally, with particular emphasis on human health
*** Electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
s
*** Fluid balance
Fluid balance is an aspect of the homeostasis of organisms in which the amount of water in the organism needs to be controlled, via osmoregulation and behavior, such that the concentrations of electrolytes (salts in solution) in the various body ...
*** Hypernatremia
Hypernatremia, also spelled hypernatraemia, is a high concentration of sodium in the blood. Early symptoms may include a strong feeling of thirst, weakness, nausea, and loss of appetite. Severe symptoms include confusion, muscle twitching, and ...
*** Hyponatremia
Hyponatremia or hyponatraemia is a low concentration of sodium in the Serum (blood), blood. It is generally defined as a sodium concentration of less than 135 mmol/L (135 mEq/L), with severe hyponatremia being below 120 mEq/L. Symp ...
*** Salt poisoning
** In plants
*** ''Arabidopsis thaliana'' responses to salinity
** In fish
*** Stenohaline fish
*** Euryhaline fish
*Salinity by geologic context
**Fresh water
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salt (chemistry), salts and other total dissolved solids. The term excludes seawater and brackish water, but it does include ...
**Seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
**Soil salinity
Soil salinity is the salt (chemistry), salt content in the soil; the process of increasing the salt content is known as salinization (also called salination in American and British English spelling differences, American English). Salts occur nat ...
**Thermohaline circulation
Thermohaline circulation (THC) is a part of the large-scale Ocean current, ocean circulation driven by global density gradients formed by surface heat and freshwater fluxes. The name ''thermohaline'' is derived from ''wikt:thermo-, thermo-'', r ...
** Paleosalinity
** CORA dataset data on salinity of global oceans
*General cases of solute concentration
** Osmotic concentration
Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L). The osmolarity of a solution is usually expressed as Osm/ ...
** Tonicity
In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two Solution (chemistry), solutions separated by a Semipermeable membrane, partially-permeable cell Cell membrane, membrane. Tonicity ...
References
Further reading
* *External links
* * * * * * (dead link) {{Authority control Chemical oceanography Aquatic ecology Oceanography Coastal geography Water quality indicators Articles containing video clips Salts