Polyfluoroalkoxyaluminates on:
[Wikipedia]
[Google]
[Amazon]
Polyfluoroalkoxyaluminates (PFAA) are
LiAlH4 + 4HOR_ -> LiAl(OR_)4 + 4H2 Reaction of lithium aluminum hydride with four equivalents of polyfluoroalcohol overnight in refluxing toluene yields the desired PFAA's . The colorless products can be precipitated from toluene in high yields on multi-gram scales by cooling at -20 °C for an hour. It can be furthered purified by sublimation.
LiAl(OR_)4 + AgF -> AgAl(OR_)4 +LiF
Ag+ l(O''hfip'')4sup>−, Ag+ l(O''hftb'')4sup>−, and Ag+ l(O''pftb'')4sup>− can be synthesized via

Li+ l(OR_)4 + 2B + HX -> (B)2 l(OR_)4 + LiX
Ab initio calculations and crystallographic structural analysis of (OEt2)2sup>+ l(O''pftb'')4sup>− indicate potential unequal sharing of the proton between the two diethyl ether molecules, and the authors propose a solid state structure in which (OEt2)2sup>+ is described as a diethyl ether molecule acting as a hydrogen bond acceptor from an ethanol molecule which stabilizes an ethyl cation as a
Li+ l(OR_)4 + NO+ bF6 -> NO+ l(OR_)4 + Li+ bF6
The NO+ l(O''pftb'')4sup>− salt can be obtained in much higher yields than the analogous ''hfpp'' salt and can be used to oxidize several transition metal and main group element complexes.
weakly coordinating anions
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found ...
many of which are of the form l(ORF)4sup>−. Most PFAA's possesses an Al(III) center coordinated by four −ORF (RF = -CPh(CF3)2 (''hfpp''), -CH(CF3)2 (''hfip''), -C(CH3)(CF3)2 (''hftb''), -C(CF3)3 (''pftb'')) ligands, giving the anion an overall -1 charge. The most weakly coordinating PFAA is an aluminate dimer, 2sup>−, which possess a bridging fluoride between two Al(III) centers. The first PFAA, l(O''hfpp'')4sup>−, was synthesized in 1996 by Steven Strauss, and several other analogs have since been synthesized, including l(O''hfip'')4sup>−, l(O''hftb'')4sup>−, and l(O''pftb'')4sup>− by Ingo Krossing in 2001. These chemically inert and very weakly coordinating ions have been used to stabilize unusual cations, isolate reactive species, and synthesize strong Brønsted acids.
Synthesis
Work by Strauss demonstrated that the synthesis of Li+ l(O''hfpp'')4sup>− could be achieved from the reaction of lithium aluminum hydride and HO''hfpp''. Analogous metal PFAA salts (MPFAA's) were later synthesized by Krossing using a similar synthetic pathway.Cation exchange and reactivity
Metal exchange
While Li+ l(O''hfpp'')4sup>− is readily soluble inhydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
solvents, presumably due to aryl substituents, Li+ l(O''hfip'')4sup>−, Li+ l(O''hftb'')4sup>−, and Li+ l(O''pftb'')4sup>− are only sparingly soluble in common organic solvents including dichloromethane (DCM), toluene, and hexane. Their silver analogs are much more soluble however, making AgPFAA's more desirable reagents for liquid phase reactivity.salt metathesis
A salt metathesis reaction (also called a double displacement reaction, double replacement reaction, or double decomposition) is a type of chemical reaction in which two ionic compounds in aqueous solution exchange their component ions to form two ...
reactions; ultrasonication of a suspension of Li+ FAAsup>− and an excess of AgF at 40 °C for 12 hours produces the final colorless products in high yields on multigram scales. Analogous M+ l(O''pftb'')4sup>−, M = Na, K, Rb, Cs, salts can also be prepared via the same synthetic route, from the metathesis reactions of Li+ l(O''pftb'')4sup>− with the corresponding MCl salt.
Brønsted acid chemistry
Strong Brønsted acids, (OEt2)2sup>+ l(O''pftb'')4sup>− and (THF)2sup>+ l(O''pftb'')4sup>− , can be prepared via the reaction of Li+ l(O''pftb'')4sup>− with two equivalents ofLewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
, Et2O or THF, and strong acid, HX (X = Cl, Br). (OEt2)2sup>+ l(O''pftb'')4sup>− is isolable as a white powder sensitive to air and water and stable at moderately high temperatures. (THF)2sup>+ l(O''pftb'')4sup>− can be isolated as a crystalline solid from a brown oily reside, presumably containing polymerized THF products formed upon addition of strong acid.

Lewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
in one resonance structure.
Nitrosonium exchange
Nitrosonium salts, NO+ l(O''hfpp'')4sup>− and NO+ l(O''pftb'')4sup>−, can be prepared via an exchange reaction of the respective lithium salt with nitrosonium hexafluoroantimonate.Cation stabilization
Transition metal complexes
Manganese(V) nitrosyl cation
The first metal nitrosyl cation was prepared using the PFAA's l(O''pftb'')4sup>− and 2sup>− as stabilizing anions.Ultraviolet radiation
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of t ...
of Mn2(CO)10 under a NO(g) atmosphere yields Mn(CO)(NO)3. Further oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of this complex is achieved through reaction with both NO+ FAAsup>−'s to yield Mn(NO)4+ FAAsup>− 's as deep red solids that are stable for months under an inert atmosphere. The Mn(NO)4+ cation is tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
and linear NO− ligand in both salts indicate 3 electron donation to the Mn(V) metal center. Rigorous tetrahedral geometry of the Mn(NO)4+ 2sup>− salt indicates a pseudo-gas phase environment about the cation due to the weakly coordinating behavior of the anionic PFAA.
Chromium(I) carbonyl radical cation
Synthesis of the chromium(I)homoleptic
In inorganic chemistry, a homoleptic chemical compound is a metal compound with all ligands identical. The term uses the " homo-" prefix to indicate that something is the same for all. Any metal species which has more than one type of ligand is he ...
radical cation, r(CO)6sup>•+, is achieved by use of PFAA's l(O''pftb'')4sup>− and 2sup>− as stabilizing anions. Oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of Cr(CO)6 by NO+ FAAsup>−'s under cold vacuum for short reaction times yields the kinetic product r(CO)6sup>•+ FAAsup>− as a pale yellow crystalline solid. Oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
in a closed room temperature vessel for long reaction times yields the thermodynamic product r(CO)5(NO)sup>+ FAAsup>− as an orange crystalline solid. Assignment of the thermodynamic and kinetic products was further supported by ab initio calculations. Fluctional Jahn-Teller distortions at room temperature are indicated by the presence of a broad band in the Raman spectra of these compounds.
Cobalt(I) sandwich complex
Cationic cobalt(I) sandwich complexes of the form Co(arene)2+ FAA can be prepared via two synthetic routes (arene =mesitylene
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenze ...
, benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, fluorobenzene
Fluorobenzene is an aryl fluoride and the simplest of the fluorobenzenes, with the formula C6H5F, often abbreviated Phenyl group, PhF. A colorless liquid, it is a precursor to many fluorophenyl compounds.
Preparation
PhF was first reported in 18 ...
, ''o''-difluorobenzene & PFAA = l(O''pftb'')4sup>− and 2sup>−). Reaction of Co(CO)5+ FAAsup>− with arene yields the cobalt(I) sandwich complex
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by hapticity, haptic, covalent bonds to two arene compound, arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ...
stabilized by a PFAA anion. Additionally, the oxidation of Co2(CO)8 with Ag+ FAAsup>− and arene yields the cobalt(I) sandwich complex stabilized by a PFAA anion and produces silver metal and gaseous carbon monoxide. Structural analysis of Co(I)bz2+ l4sup>− reveals the sandwich complex is slightly staggered, twisted 6° from an eclipsed confirmation. 3° bending of C-H bonds towards the cobalt center yields D6 symmetry. The cobalt sandwich complex can be used as a precursor to synthesize Co(PtBu3)2 upon ligand substitution.
Nickel(I) complexes
Oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of Ni(COD)2 with Ag+ l(O''pftb'')4sup>− yields Ni(COD)2+ l(O''pftb'')4sup>− as an orange crystalline solid. In the solid phase the material is stable to air and moisture, but is sensitive to diatomic oxygen in solution. EPR analysis reveals that 90% of the unpaired electron spin density is located on the nickel center. This nickel salt serves as synthetically feasible precursor to a series of nickel(I) arene
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
and phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
cations stabilized by PFAA's. Reactions of Ni(COD)2+ l(O''pftb'')4sup>− with mesitylene
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenze ...
, benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, or hexamethyl benzene results in substitution of one COD ligand. Arene ligand exchange results in partial electron spin delocalization onto the aromatic arene ligand, with 84-87% of the unpaired electron spin density located on the nickel center. Reactions of Ni(COD)2+ l(O''pftb'')4sup>− with phosphines results in complete ligand substitution and dissociation of COD
Cod (: cod) is the common name for the demersal fish genus ''Gadus'', belonging to the family (biology), family Gadidae. Cod is also used as part of the common name for a number of other fish species, and one species that belongs to genus ''Gad ...
. Addition of chelating
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
phosphines, 1,3-bis(diphenylphosphino)propane
1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula PhP(CH)PPh. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is ...
(dppp) and 1,2-bis(diphenylphosphino)propane (dppe) yields four coordinate distorted tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
nickel cations. Addition of triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a l ...
yields a three coordinate trigonal planar
In chemistry, trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of an equilateral triangle, called peripheral atoms, all in one plane. In an ideal trigonal planar species, all three ligands a ...
cation. Addition of bulky tri-tert-butylphosphine yields a two coordinate linear cation.
Main group element complexes

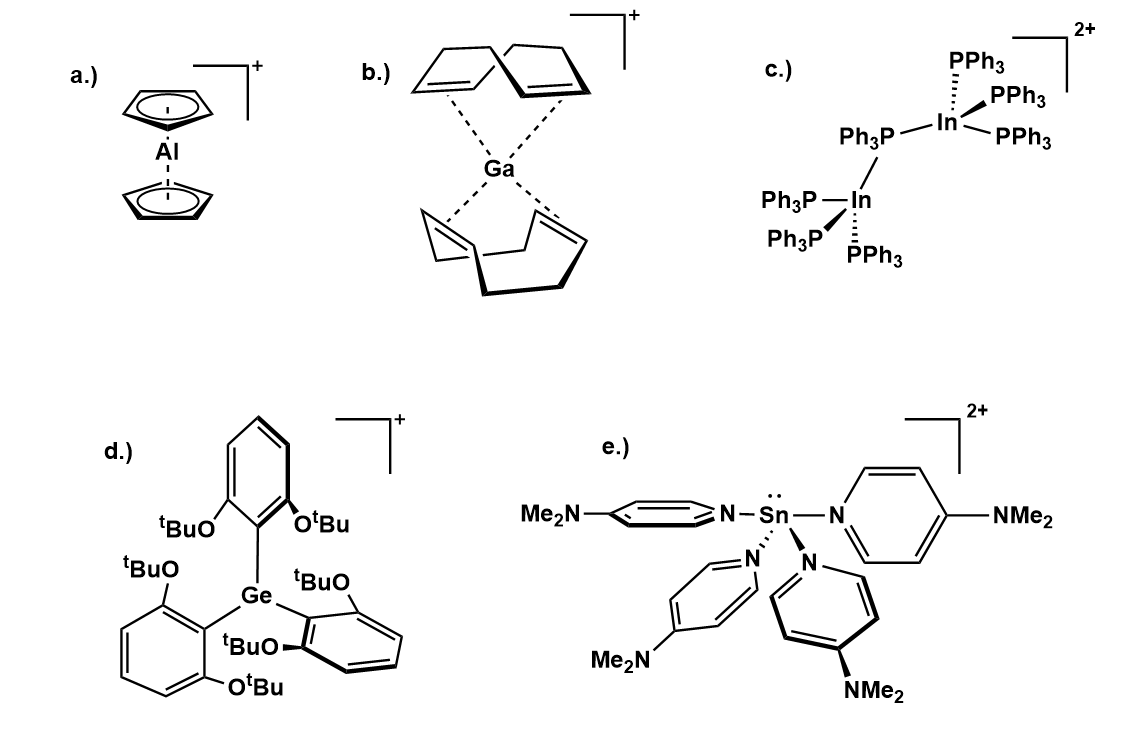
AlCp2+
Reaction of AlCp3 with the strong Brønsted acid, (OEt2)2sup>+ l(O''pftb'')4sup>−, yields lCp2sup>+ l(O''pftb'')4sup>− as colorless solid as well as lCp2•2Et2Osup>+ l(O''pftb'')4sup>−. The former complex exhibits nearly identical bonding to its analog AlCp*2+ while the Cp substituents in the later compound exhibit η1 bonding due to twodiethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
substituents bound to the aluminum center.
Gallium(I) olefin complex
The first main-grouphomoleptic
In inorganic chemistry, a homoleptic chemical compound is a metal compound with all ligands identical. The term uses the " homo-" prefix to indicate that something is the same for all. Any metal species which has more than one type of ligand is he ...
olefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
compound isolable in bulk was synthesized using a stabilizing PFAA counter ion. a(PhF)2sup>+ l(O''pftb'')4sup>− can be prepared via the oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of Ga by Ag+ l(O''pftb'')4sup>− in the presence of fluorobenzene
Fluorobenzene is an aryl fluoride and the simplest of the fluorobenzenes, with the formula C6H5F, often abbreviated Phenyl group, PhF. A colorless liquid, it is a precursor to many fluorophenyl compounds.
Preparation
PhF was first reported in 18 ...
. Fluorobenzene
Fluorobenzene is an aryl fluoride and the simplest of the fluorobenzenes, with the formula C6H5F, often abbreviated Phenyl group, PhF. A colorless liquid, it is a precursor to many fluorophenyl compounds.
Preparation
PhF was first reported in 18 ...
ligands can then be displaced by COD
Cod (: cod) is the common name for the demersal fish genus ''Gadus'', belonging to the family (biology), family Gadidae. Cod is also used as part of the common name for a number of other fish species, and one species that belongs to genus ''Gad ...
to produce a(COD)2sup>+ l(O''pftb'')4sup>−. AIM
AIM or Aim may refer to:
Computing
* AIM alliance, an Apple-IBM-Motorola alliance
* AIM (software), AOL Instant Messenger
* Fortyfive, a Japanese software development company previously known as AIM
Military
* Abrams Integrated Management, an ...
analysis of the compound reveals minimal back bonding to the olefin double bonds, characterizing the ligand-Ga interactions as primarily electrostatic. The gallium salt serves as a precursor to gallium phosphine complexes, as addition of triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a l ...
yields a(PPh3)2sup>+ l(O''pftb'')4sup>−.
Germyl cation
Halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
abstraction from BrGeR3 (R = 6H3(OtBu)2germyl
Germyl, trihydridogermanate(1-), trihydrogermanide, trihydridogermyl or according to IUPAC Red Book: germanide is an anion containing germanium bounded with three hydrogens, with formula . Germyl is the IUPAC term for the – group. For less elect ...
cation Ge 6H3(OtBu)2germyl
Germyl, trihydridogermanate(1-), trihydrogermanide, trihydridogermyl or according to IUPAC Red Book: germanide is an anion containing germanium bounded with three hydrogens, with formula . Germyl is the IUPAC term for the – group. For less elect ...
cation and the PFAA, giving rise to a very electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
germanium species.
Tin(II) dications
Various tin(II) dications can be synthesized with PFAA's as counterions. n(MeCN)6sup>2+ l(O''pftb'')4sub>2− can be prepared via the oxidation of tin metal with NO+ l(O''pftb'')4sup>−. Addition ofpyrazine
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine. It is a ''"deliquescent crystal or wax-lik ...
to this complex results in ligand substitution to produce n(pyz)2(MeCN)4sup>2+, while addition of triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a l ...
produces n(PPh3)2(MeCN)4sup>2+•MeCN. The salt, Sn(dmap)42+ l(O''pftb'')4sub>2− is prepared by a different synthetic route. Halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
abstraction of SnCpCl by Li+ l(O''pftb'')4sup>− yields nCpsup>+ which produces Sn(dmap)42+ upon addition of dmap. Sn(dmap)42+ adopts a see-saw geometry with dmap ligands stabilizing a Sn(II) center.
P9+
The cationic P9+ cluster can be isolated from the oxidation of P4 by NO+ l(O''pftb'')4sup>−. In the multistep reaction, 4NOsup>+ is a proposed intermediate from analysis ofcollision-induced dissociation
Collision-induced dissociation (CID), also known as collisionally activated dissociation (CAD), is a mass spectrometry technique to induce fragmentation (chemistry), fragmentation of selected ions in the gas phase. The selected ions (typically m ...
(CID) experiments. Complex coupling present in the 31P NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
spectra of P9+ allowed for the determination of its structure.
Applications
Ionic liquids
Due to lowpolarizability
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of particles with an electric charge. When subject to an elect ...
, large charge delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly diff ...
, and high conformational flexibility, PFAA salts are potentially useful ionic liquid
An ionic liquid (IL) is a salt (chemistry), salt in the liquid state at ambient conditions. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as wate ...
s.{{Cite journal, last1=Rupp, first1=Alexander B. A., last2=Krossing, first2=Ingo, date=2015-09-15, title=Ionic Liquids with Weakly Coordinating III(ORF)4ˆ’ Anions, url=https://doi.org/10.1021/acs.accounts.5b00247, journal=Accounts of Chemical Research, volume=48, issue=9, pages=2537–2546, doi=10.1021/acs.accounts.5b00247, pmid=26299782, issn=0001-4842, url-access=subscription Several PFAA salts, including those of l(O''hfip'')4sup>−, possess melting points as low as 273 K or colder. Walden Plots, which are created by plotting the logarithm of conductivity against the logarithm of inverse viscosity, indicate that several l(O''hfip'')4sup>− ionic liquids are potentially better than the best commercially available ionic liquids. Better ionic liquids are defined to have high conductivities and high viscosities
Viscosity is a measure of a fluid's rate-dependent resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for example, syrup h ...
.
See also
*Non-coordinating anion
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly foun ...
s
* Ionic liquid
An ionic liquid (IL) is a salt (chemistry), salt in the liquid state at ambient conditions. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as wate ...
s
References