Poly(trimethylene Carbonate) on:
[Wikipedia]
[Google]
[Amazon]
Poly(trimethylene carbonate) (PTMC) is an aliphatic
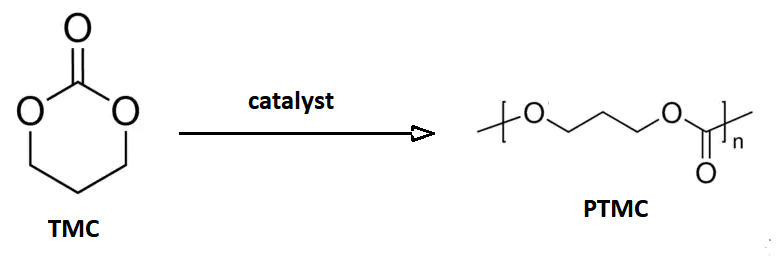
polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate ester, carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, toughness, tough materials, and some grades are optically transp ...
synthesized from the 6-membered cyclic carbonate, trimethylene carbonate
Trimethylene carbonate, or 1,3-propylene carbonate, is a 6-membered cyclic carbonate ester. It is a colourless solid that upon heating or catalytic ring-opening converts to poly(trimethylene carbonate) (PTMC). Such polymers are called aliphatic p ...
(1,3-propylene carbonate or 1,3-Dioxan-2-one). Trimethylene carbonate (TMC) is a colorless crystalline solid with melting point ranging between 45°C and 48 °C and boiling point at 255°C (at 760 mmHg). TMC is originally synthesized from 1,3-propanediol with phosgene
Phosgene is an organic chemical compound with the formula . It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. It can be thought of chemically as the double acyl chloride analog of ...
or carbon monoxide, which are highly poisonous gases. Another route is from the transesterification
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the r ...
of 1,3-propanediol and dialkylcarbonates. This route is considered "greener" compared to the other one, since precursors can be obtained from renewable resources and carbon dioxide.
Synthesis
In opposition to five-membered cyclic carbonate, the six-membered ones like trimethylene carbonate are thermodynamically less stable than its polymer, undergoing the ring-opening polymerization with retention of CO in the polymer structure, and generation an aliphatic polycarbonate.
Ring-opening polymerization
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anion ...
(ROP) is the most common method used to synthesize poly(trimethylene carbonate) and their copolymers, since this synthetic route can allow mild reaction condition.
Several ROP catalysts/initiators have been used to synthesize the polymer, among them metal-catalyzed polymerization using oxides, salts and complexes of Al, K, Ti, Zn, Zr, Sn and rare earths metals; enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
-catalyzed polymerization; and alcohol-initiated polymerization.
Physical properties
PTMC is a predominantly amorphous polymer in the relaxed state but it can present some crystallinity, particularly when the chains are stretched. The polymer presentsglass transition temperature
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rub ...
() between -15 and -30 °C and melting temperature () ranging from 38 to 41°C.
Low molecular weight PTMC is a rubbery polymer with poor dimensional stability, tackiness, and inadequate mechanical properties. Nevertheless, high molecular weight amorphous PTMC (over 100,000) is very flexible, with a relatively low elastic modulus (5–7 MPa) at room temperature, tough and it presents excellent ultimate mechanical properties. Mechanical properties of the rubber can be also improved upon cross-linking by gamma-irradiation.
PTMC has resistance to non-enzymatic hydrolysis, compared to most aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all ...
polyesters
Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include some natura ...
, but it is biodegradable ''in vivo'' by enzymes. It is a resorbable material since the ester bonds can be enzymatically broken, producing CO and water. So, ''in vivo'', it degrades by surface erosion and its decomposition products contain no organic acids, preventing potential inflammatory responses.
Applications
Due to the predominant amorphous nature, PTMC is a flexible polymer with rubbery behavior. In addition, thebiodegradability
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegrada ...
and biocompatibility
Biocompatibility is related to the behavior of biomaterials in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoin ...
of PTMC make it to have high applicability in biomedical applications as scaffolds for tissue regeneration and drug delivery devices.
PTMC has been used as scaffolds for tissue engineering
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biolo ...
particularly for some types of soft tissues in which the maintenance of mechanical properties is important for tissue reconstruction. PTMC-based membranes have been also evaluated as barrier for use in hard tissue guided regeneration like bone. The performance of these membranes is comparable with commercial collagen and e-PTFE membranes, showing well suitability for use in guided bone regeneration.
Due to rubbery and hydrophobic nature, PTMC-based copolymers produced from ROP of TMC with lactone
Lactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated.
Lactones are formed by lactonization, the intramolecular esterification of the corresp ...
-based comonomer
In polymer chemistry, a comonomer refers to a polymerizable precursor to a copolymer aside from the principal monomer. In some cases, only small amounts of a comonomer are employed, in other cases substantial amounts of comonomers are used. Furt ...
s have been synthesized to modify these characteristics, amplifying applications. Thus, the use as resorbable medical devices in which control of rigidity and biodegradation time are desired has been proposed. Main examples of these copolymers are poly(L-lactide-co-trimethylene carbonate), poly(glycolide-''co''-trimethylene carbonate) and poly(caprolactone-''co''-trimethylene carbonate).{{Cite journal , last1=Rocha , first1=Daniela Nogueira , last2=Brites , first2=Pedro , last3=Fonseca , first3=Carlos , last4=Pêgo , first4=Ana Paula , date=Feb 2014 , title=Poly(Trimethylene Carbonate-co-ε-Caprolactone) Promotes Axonal Growth , journal=PLOS ONE , language=en , volume=9 , issue=2 , pages=e88593 , doi=10.1371/journal.pone.0088593 , issn=1932-6203 , pmc=3937290 , pmid=24586346, bibcode=2014PLoSO...988593R , doi-access=free
Poly(L-lactide-co-trimethylene carbonate) has been proposed for application as small diameter vascular grafts. Poly(glycolide-''co''-trimethylene carbonate) is a commercial monofilament used for suture with slow biodegradation rate which allows maintenance of high mechanical strength compatible with the surgical recovery. Poly(caprolactone-''co''-trimethylene carbonate) has been proposed as biomaterial for conduits in the regeneration of central nervous system.
References