|
Trimethylene Carbonate
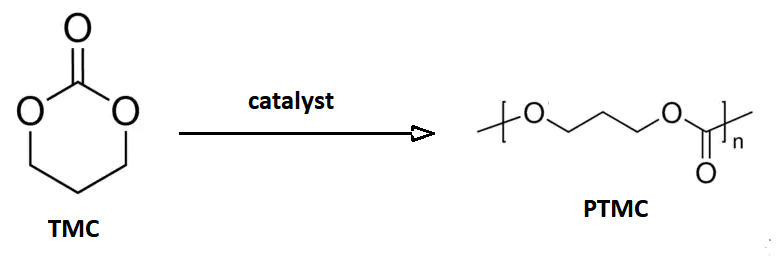
Trimethylene carbonate, or 1,3-propylene carbonate, is a 6-membered cyclic carbonate ester. It is a colourless solid that upon heating or catalytic ring-opening converts to poly(trimethylene carbonate) (PTMC). Such polymers are called aliphatic polycarbonates and are of interest for potential biomedical applications. An isomeric derivative is propylene carbonate, a colourless liquid that does not spontaneously polymerize. Preparation This compound may be prepared from 1,3-propanediol and ethyl chloroformate (a phosgene substitute), or from oxetane and carbon dioxide with an appropriate catalyst: :HOC3H6OH + ClCO2C2H5 → C3H6O2CO + C2H5OH + HCl :C3H6O + CO2 → C3H6O2CO This cyclic carbonate undergoes ring-opening polymerization to give poly(trimethylene carbonate), abbreviated PTMC. : Medical devices The polymer PTC is of commercial interest as a biodegradable polymer with biomedical applications. A block copolymer of glycolic acid and trimethylene carbonate (TMC) is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Ester
In organic chemistry, a carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is and they are related to esters (), ethers () and also to the inorganic carbonates. Monomers of polycarbonate (e.g. Makrolon or Lexan) are linked by carbonate groups. These polycarbonates are used in eyeglass lenses, compact discs, and bulletproof glass. Small carbonate esters like dimethyl carbonate, ethylene carbonate, propylene carbonate are used as solvents, dimethyl carbonate is also a mild methylating agent. Structures Carbonate esters have planar OC(OC)2 cores, which confers rigidity. The unique O=C bond is short (1.173 Å in the depicted example), while the C–O bonds are more ether-like (the bond distances of 1.326 Å for the example depicted). Carbonate esters can be divided into three structural classes: acyclic, cyclic, and polym ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poly(trimethylene Carbonate)
Poly(trimethylene carbonate) (PTMC) is an aliphatic polycarbonate synthesized from the 6-membered cyclic carbonate, trimethylene carbonate (1,3-propylene carbonate or 1,3-Dioxan-2-one). Trimethylene carbonate, Trimethylene carbonate (TMC) is a colorless crystalline solid with melting point ranging between 45°C and 48 °C and boiling point at 255°C (at 760 mmHg). TMC is originally synthesized from 1,3-Propanediol, 1,3-propanediol with phosgene or carbon monoxide, which are highly poisonous gases. Another route is from the transesterification of 1,3-propanediol and Dialkyl carbonate, dialkylcarbonates. This route is considered "greener" compared to the other one, since precursors can be obtained from renewable resources and carbon dioxide. Synthesis In opposition to five-membered cyclic carbonate, the six-membered ones like trimethylene carbonate are thermodynamically less stable than its polymer, undergoing the ring-opening polymerization with retention of CO_2 in the polymer s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propylene Carbonate
Propylene carbonate (often abbreviated PC) is an organic compound with the formula C4H6O3. It is a cyclic carbonate ester derived from propylene glycol. This colorless and odorless liquid is useful as a polar, aprotic solvent. Propylene carbonate is chiral, but is used as the racemic mixture in most contexts. Preparation Although many organic carbonates are produced using phosgene, propylene and ethylene carbonates are exceptions. They are mainly prepared by the carbonation of the epoxides (epoxypropane, or propylene oxide here): :CH3CHCH2O + CO2 → CH3C2H3O2CO The corresponding reaction of 1,2-propanediol with phosgene is complex, yielding not only propylene carbonate but also oligomeric products. Propylene carbonate can also be synthesized from urea and propylene glycol over zinc acetate. Applications As a solvent Propylene carbonate is used as a polar, aprotic solvent. It has a high molecular dipole moment (4.9 D), considerably higher than those of acetone (2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Chloroformate
Ethyl chloroformate is an organic compound with the chemical formula . It is the ethyl ester of chloroformic acid. It is a colorless, corrosive and highly toxic liquid. It is a reagent used in organic synthesis for the introduction of the ethyl carbamate protecting group and for the formation of carboxylic anhydrides. Preparation Ethyl chloroformate can be prepared using ethanol and phosgene Phosgene is an organic chemical compound with the formula . It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. It can be thought of chemically as the double acyl chloride analog of ...: : Safety Ethyl chloroformate is a highly toxic, flammable, corrosive substance. It causes severe burns when comes in contact with eyes and/or skin, can be harmful if swallowed or inhaled. References {{Authority control Chloroformates Reagents for organic chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosgene
Phosgene is an organic chemical compound with the formula . It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. It can be thought of chemically as the double acyl chloride analog of carbonic acid, or structurally as formaldehyde with the hydrogen atoms replaced by chlorine atoms. In 2013, about 75–80 % of global phosgene was consumed for isocyanates, 18% for polycarbonates and about 5% for other fine chemicals. Phosgene is extremely poisonous and was used as a chemical weapon during World War I, where it was responsible for 85,000 deaths. It is a highly potent pulmonary irritant and quickly filled enemy trenches due to it being a heavy gas. It is classified as a Schedule 3 substance under the Chemical Weapons Convention. In addition to its industrial production, small amounts occur from the breakdown and the combustion of organochlorine compounds, such as chloroform. Structure and basic properties Phosgene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxetane
Oxetane, or 1,3-propylene oxide, is a heterocycle, heterocyclic organic compound with the molecular formula , having a four-membered ring with three carbon atoms and one oxygen atom. The term "an oxetane" or "oxetanes" refer to any organic compound containing the oxetane ring. Production A typical well-known method of preparation is the reaction of potassium hydroxide with 3-chloropropyl acetate at 150 °C: Yield of oxetane made this way is c. 40%, as the synthesis can lead to a variety of by-products including water, potassium chloride, and potassium acetate. Another possible reaction to form an oxetane ring is the Paternò–Büchi reaction. The oxetane ring can also be formed through diol cyclization as well as through decarboxylation of a six-membered cyclic carbonate. Derivatives More than a hundred different oxetanes have been synthesized. Functional groups can be added into any desired position in the oxetane ring, including fully fluorinated (perfluorinated) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TCM Polymerization
TCM may refer to: Arts and music Film * ''The Texas Chainsaw Massacre'' (franchise), a horror film franchise ** ''The Texas Chain Saw Massacre'', the original 1974 film ** ''The Texas Chainsaw Massacre'' (2003 film), the 2003 remake Games * ''The Corporate Machine'', a 2001 personal computer game from Stardock * ''Total Club Manager'', football (soccer) management series of video games from Bright Future (in older releases EA sports) Music * The Color Morale, a post-hardcore band from Rockford, Illinois * The Crystal Method, an electronic music group * Tianjin Conservatory of Music * Tokyo College of Music * Trinity College of Music, a leading music conservatory, based in Greenwich, London, United Kingdom Automotive * Transmission control module, an electronic device to manage an automobile's transmission Companies * Teledyne Continental Motors, an American engine manufacturing company * ''Times Community Media'', an American newspaper company * Toshiba Consumer Marketing C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolic Acid
Glycolic acid (or hydroxyacetic acid; chemical formula ) is a colorless, odorless and hygroscopic crystal, crystalline solid, highly solubility, soluble in water. It is used in various skin care, skin-care products. Glycolic acid is widespread in nature. A glycolate (sometimes spelled "glycollate") is a Salt (chemistry), salt or ester of glycolic acid. History The name "glycolic acid" was coined in 1848 by French chemist Auguste Laurent (1807–1853). He proposed that the amino acid glycine—which was then called ''glycocolle''—might be the amine of a hypothetical acid, which he called "glycolic acid" (''acide glycolique''). Glycolic acid was first prepared in 1851 by German chemist Adolph Strecker (1822–1871) and Russian chemist Nikolai Nikolaevich Sokolov (1826–1877). They produced it by treating hippuric acid with nitric acid and nitrogen dioxide to form an ester of benzoic acid and glycolic acid (), which they called "benzoglycolic acid" (''Benzoglykolsäure''; also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surgical Suture
A surgical suture, also known as a stitch or stitches, is a medical device used to hold Tissue (biology), body tissues together and approximate wound edges after an injury or surgery. Application generally involves using a Sewing needle, needle with an attached length of thread (yarn), thread. There are numerous types of suture which differ by needle shape and size as well as thread material and characteristics. Selection of surgical suture should be determined by the characteristics and location of the wound or the specific body tissues being approximated. In selecting the needle, thread, and suturing technique to use for a specific patient, a medical care provider must consider the tensile strength of the specific suture thread needed to efficiently hold the tissues together depending on the mechanical and shear forces acting on the wound as well as the thickness of the tissue being approximated. One must also consider the elasticity of the thread and ability to adapt to differe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Carbonate
Ethylene carbonate (sometimes abbreviated EC) is the organic compound with the formula (CH2O)2CO. It is classified as the cyclic carbonate ester of ethylene glycol and carbonic acid. At room temperature (25 °C) ethylene carbonate is a transparent crystalline solid, practically odorless and colorless, and somewhat soluble in water. In the liquid state (m.p. 34-37 °C) it is a colorless odorless liquid. JEFFSOL ETHYLENE CARBONATE catalog entry at www.huntsman.com. Accessed on 2010-02-18. Production and reactions Ethylene carbonate is produced by the reaction between and |