Piano Stool Complex on:
[Wikipedia]
[Google]
[Amazon]
Half sandwich compounds, also known as piano stool complexes, are
MMT-2D-skeletal.png, MMT is a commercially useful antiknock compound.
Cpco(CO)2.png, CpCo(CO)2 is a catalyst for the synthesis of pyridines.
Cyclobutadiene-iron-tricarbonyl-from-xtal-3D-balls.png, (C4H4)Fe(CO)3.
Cp2Fe(CO)2I-2D-skeletal.png, CpFe(CO)2I is an example of a piano stool complex with two different monodentate ligands.
RuCymCl2.png, The diruthenium of cymene is readily cleaved by ligands to give monoRu half-sandwich derivatives.
CHTMo(CO)3.png, Cycloheptatriene molybdenum tricarbonyl
CPPCDV01.png, Cp2V2(CO)5 featuring a pair of semi-bridging CO ligands.


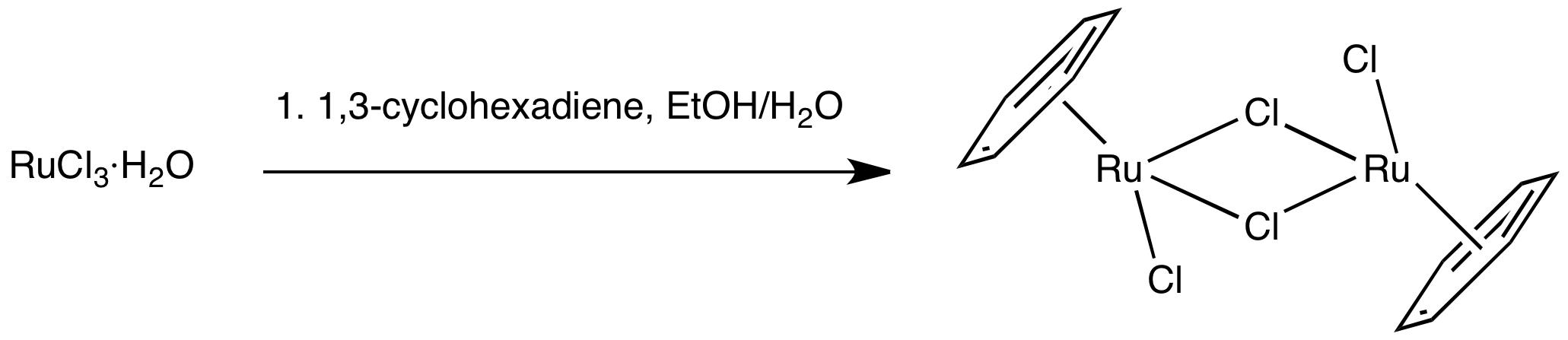 (''η''6-C6H6)RuCl2 readily undergoes ligand exchange via cleavage of the chloride bridges, making this complex a versatile precursor to Ru(II) piano stool derivatives.
(''η''6-C6H6)RuCl2 readily undergoes ligand exchange via cleavage of the chloride bridges, making this complex a versatile precursor to Ru(II) piano stool derivatives.
organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
complexes that feature a cyclic polyhapto ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol
Gasoline (North American English) or petrol ( Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When formul ...
.
(''η''5-C5H5) piano stool compounds
Half sandwich complexes containing cyclopentadienyl ligands are common. Well studied examples include (''η''5-C5H5)V(CO)4, (''η''5-C5H5)Cr(CO)3H, (''η''5-CH3C5H4)Mn(CO)3, (''η''5-C5H5)Cr(CO)3H, ''η''5-C5H5)Fe(CO)3sup>+, (''η''5-C5H5)V(CO)4I, and (''η''5-C5H5)Ru(NCMe). (''η''5-C5H5)Co(CO)2 is a two-legged piano stool complex. Bulky cyclopentadienyl ligands such as 1,2,4-C5H2(''tert''-Bu)3− form unusual half-sandwich complexes.(''η''6-C6H6) piano stool compounds
Inorganometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
, (''η''6-C6H6) piano stool compounds are half-sandwich compounds with (''η''6-C6H6)ML3 structure (M = Cr, Mo, W, Mn(I), Re(I) and L = typically CO). (''η''6-C6H6) piano stool complexes are stable 18-electron coordination compounds with a variety of chemical and material applications. Early studies on (''η''6-C6H6)Cr(CO)3 were carried out by Natta, Ercoli and Calderazzo, and Fischer and Ofele, and the crystal structure was determined by Corradini and Allegra in 1959. The X-ray data indicate that the plane of the benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
ring is nearly parallel to the plane defined by the oxygen atoms of the carbonyl ligands, and so the structure resembles a benzene seat mounted on three carbonyl legs tethered by the metal atom.
Cr and Mn(I) (''η''6-C6H6) piano stool complexes
Piano stool complexes of the type (''η''6-C6H6)M(CO)3 are typically synthesized by heating the appropriate metal carbonyl compound withbenzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
. Alternately, the same compounds can be obtained by carbonylation of the bis(arene) sandwich compounds, such as (''η''6-C6H6)2M compound with the metal carbonyl compound. This second approach may be more appropriate for arene ligands containing thermally fragile substituents.
:
Reactivity of (''η''6-C6H6)Cr(CO)3
The benzene ligand in (''η''6-C6H6)Cr(CO)3 is prone to deprotonation. For example, Organolithium compounds form adducts featuring cyclohexadienyl ligands. Subsequentoxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of the complex results in the release of a substituted benzene. Oxidation of the chromium atom by I2 and other iodine reagents has been shown to promote exchange of arene ligands, but the intermediate chromium iodide species has not been characterized.
:
(''η''6-C6H6)Cr(CO)3 complexes exhibit "''cine''" and "''tele''" nucleophilic aromatic addition. Processes of this type involve reaction of (''η''6-C6H6)Cr(CO)3 with an alkyl lithium reagent. Subsequent treatment with an acid results in the addition of a nucleophile to the benzene ring at a site ''ortho'' ("''cine''"), ''meta'' or ''para'' ("''tele''") to the ''ipso'' carbon (see Arene substitution patterns).
:
Reflecting its increased acidity, the benzene ligand can be lithiated with ''n''-butyllithium. The resulting organolithium compound serves as a nucleophile in various reactions, for example, with trimethylsilyl chloride:
:
(''η''6-C6H6)Cr(CO)3 is a useful catalyst for the hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
of 1,3-diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nome ...
s. The product alkene results from 1,4-addition of hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
. The complex does not hydrogenate isolated double bonds.
A variety of arenes ligands have been installed aside from benzene. Weakly coordinating ligands may be employed to improve ligand exchange and thus the turnover rates for (''η''6-C6H6)M(CO)3 complexes.(''η''6-C6H6)M(CO)3 complexes have been incorporated into high surface area porous materials.
(''η''6-C6H6)M(CO)3 complexes serve as models for the interaction of metal carbonyls with graphene and carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range ( nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
* ''Single-walled carbon nanotubes'' (''S ...
s. The presence of M(CO)3 on extended π-network materials has been shown to improve electrical conductivity across the material.
Reactivity of ''η''6-C6H6)Mn(CO)3sup>+
Typical arene tricarbonyl piano stool complexes of Mn(I) and Re(I) are cationic and thus exhibit enhanced reactivity toward nucleophiles. Subsequent to nucleophilic addition, the modified arene can be recovered from the metal. :
(''η''6-C6H6)Ru complexes
Half-sandwich compounds employing Ru(II), such as (cymene)ruthenium dichloride dimer, have been mainly investigated as catalysts for transfer hydrogenation. These complexes feature three coordination sites that are susceptible to substitution, while the arene ligand is tightly bonded and protects the metal against oxidation to Ru(III). They are prepared by reaction of RuCl3·''x''(H2O) with 1,3-cyclohexadienes. Work is also conducted on their potential as anticancer drugs. : (''η''6-C6H6)RuCl2 readily undergoes ligand exchange via cleavage of the chloride bridges, making this complex a versatile precursor to Ru(II) piano stool derivatives.
(''η''6-C6H6)RuCl2 readily undergoes ligand exchange via cleavage of the chloride bridges, making this complex a versatile precursor to Ru(II) piano stool derivatives.
References
{{organometallics Organic compounds Organometallic chemistry Coordination chemistry