Photolabile Protecting Group on:
[Wikipedia]
[Google]
[Amazon]
A photolabile protecting group (PPG; also known as: photoremovable, photosensitive, or photocleavable protecting group) is a chemical modification to a
 The first reported use of a PPG in the
The first reported use of a PPG in the
 Nitrobenzyl-based PPGs are often considered the most commonly used PPGs. These PPGs are traditionally identified as Norrish Type II reaction as their
Nitrobenzyl-based PPGs are often considered the most commonly used PPGs. These PPGs are traditionally identified as Norrish Type II reaction as their
 Additional modifications have targeted the
Additional modifications have targeted the
 The phenacyl PPG is the archetypal example of a carbonyl-based PPG. Under this motif, the PPG is attached to the protected substrate at the αβ-carbon, and can exhibit varied photodeprotection mechanisms based on the phenacyl skeleton, substrate identify and reaction conditions. Overall, phenacyl PPGs can be used to protect
The phenacyl PPG is the archetypal example of a carbonyl-based PPG. Under this motif, the PPG is attached to the protected substrate at the αβ-carbon, and can exhibit varied photodeprotection mechanisms based on the phenacyl skeleton, substrate identify and reaction conditions. Overall, phenacyl PPGs can be used to protect
 Barltrop and Schofield first demonstrated the use of a benzyl-based PPG, structural variations have focused on substitution to the
Barltrop and Schofield first demonstrated the use of a benzyl-based PPG, structural variations have focused on substitution to the

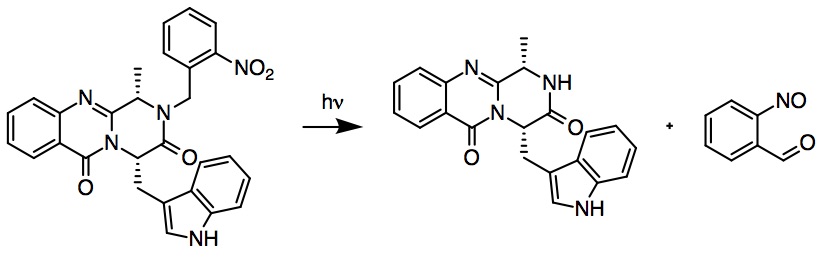
 Protecting a substrate with a PPG is commonly referred to as "photocaging." This term is especially popular in biological systems. For example, Ly ''et al.'' developed a ''p''-iodobenzoate-based photocaged reagent, which would experience a homolytic photoclevage of the C-I bond. They found that the reaction could occur with excellent yields, and with a
Protecting a substrate with a PPG is commonly referred to as "photocaging." This term is especially popular in biological systems. For example, Ly ''et al.'' developed a ''p''-iodobenzoate-based photocaged reagent, which would experience a homolytic photoclevage of the C-I bond. They found that the reaction could occur with excellent yields, and with a 
 When
When
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
that can be removed with light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 te ...
. PPGs enable high degrees of chemoselectivity
Chemoselectivity is the preferential outcome of a chemical reaction over a set of possible alternative reactions.
In another definition, chemoselectivity refers to the selective reactivity of one functional group in the presence of others; often ...
as they allow researchers to control spatial, temporal and concentration variables with light. Control of these variables is valuable as it enables multiple PPG applications, including orthogonality in systems with multiple protecting groups. As the removal of a PPG does not require chemical reagents
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
, the photocleavage of a PPG is often referred to as "traceless reagent processes", and is often used in biological model systems and multistep organic syntheses. Since their introduction in 1962, numerous PPGs have been developed and utilized in a variety of wide-ranging applications from protein science to photoresists. Due to the large number of reported protecting groups, PPGs are often categorized by their major functional group(s); three of the most common classifications are detailed below.
Historical introduction
 The first reported use of a PPG in the
The first reported use of a PPG in the scientific literature
: ''For a broader class of literature, see Academic publishing.''
Scientific literature comprises scholarly publications that report original empirical and theoretical work in the natural and social sciences. Within an academic field, sci ...
was by Barltrop and Schofield, who in 1962 used 253.7 nm light to release glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinog ...
from N-benzylglycine. Following this initial report, the field rapidly expanded throughout the 1970s as Kaplan and Epstein studied PPGs in a variety of biochemical systems. During this time, a series of standards for evaluating PPG performance was compiled. An abbreviated list of these standards, which are commonly called the Lester rules, or Sheehan criteria are summarized below:
* In biological systems, the protected substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (locomotion), the surface over which an organism lo ...
, as well as the photoproducts should be highly soluble in water; in synthesis, this requirement is not as strict
* The protected substrate, as well as the photoproducts should be stable in the photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
environment
* Separation of the PPG should exhibit a quantum yield The quantum yield (Φ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
Applications
Fluorescence spectroscopy
The fluorescence quantum yield is defined as the ratio of the numb ...
greater than 0.10
* Separation of the PPG should occur through a primary photochemical
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400– ...
process
* The chromophore
A chromophore is the part of a molecule responsible for its color.
The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molec ...
should absorb incident light
In optics a ray is an idealized geometrical model of light, obtained by choosing a curve that is perpendicular to the ''wavefronts'' of the actual light, and that points in the direction of energy flow. Rays are used to model the propagation o ...
with reasonable absorptivity
* The excitation wavelength
Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating fi ...
of light should be greater than 300 nm
* The media
Media may refer to:
Communication
* Media (communication), tools used to deliver information or data
** Advertising media, various media, content, buying and placement for advertising
** Broadcast media, communications delivered over mass el ...
and photoproducts should not absorb the incident light
* A general, high-yield synthetic procedure should exist for attaching the PPG to an unprotected substrate
* The protected substrate and the photoproducts should be easily separated
Main classifications
Nitrobenzyl-based PPGs
Norrish Type II mechanism
 Nitrobenzyl-based PPGs are often considered the most commonly used PPGs. These PPGs are traditionally identified as Norrish Type II reaction as their
Nitrobenzyl-based PPGs are often considered the most commonly used PPGs. These PPGs are traditionally identified as Norrish Type II reaction as their mechanism
Mechanism may refer to:
*Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission
*Mechanism (biology), explaining how a feature is created
*Mechanism (philosophy), a theory that a ...
was first described by Norrish in 1935. Norrish elucidated that an incident photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are Massless particle, massless ...
(200 nm < λ < 320 nm) breaks the N=O π-bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
in the nitro-group, bringing the protected substrate into a diradical
In chemistry, a diradical is a molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate. The term "diradical" is mainly used to describe organic compounds, where most diradicals are extremely reactive and i ...
excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers t ...
. Subsequently, the nitrogen radical
Radical may refer to:
Politics and ideology Politics
* Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe an ...
abstracts a proton from the benzylic
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substi ...
carbon, forming the ''aci''-nitro compound. Depending on pH, solvent and the extent of substitution, the aci-nitro intermediate decays at a rate of roughly 102–104 s−1. Following resonance of the π-electrons, a five-membered ring is formed before the PPG is cleaved yielding 2-nitrosobenzaldehyde and a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
.
Overall, nitrobenzyl-based PPGs are highly general. The list of functional groups that can be protected include, but are not limited to, phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
s, carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carbox ...
s, carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonat ...
s, carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formall ...
s, thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
ates, phenolates
Phenolates (also called phenoxides) are anions, salts, and esters of phenols. They may be formed by reaction of phenols with strong base.
Properties
Alkali metal phenolates, such as sodium phenolate hydrolyze in aqueous solution to form basic ...
and alkoxides. Additionally, while the rate varies with a number of variables, including choice of solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
and pH, the photodeprotection has been exhibited in both solution and in the solid-state
Solid state, or solid matter, is one of the four fundamental states of matter.
Solid state may also refer to:
Electronics
* Solid-state electronics, circuits built of solid materials
* Solid state ionics, study of ionic conductors and their ...
. Under optimal conditions, the photorelease can proceed with >95% yield. Nevertheless, the photoproducts of this PPG are known to undergo imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
formation when irradiated at wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tr ...
s above 300 nm. This side product
A by-product or byproduct is a secondary product derived from a production process, manufacturing process or chemical reaction; it is not the primary product or service being produced.
A by-product can be useful and marketable or it can be consid ...
often competes for incident radiation, which may lead to decreased chemical and quantum yields.
Common modifications
In attempts to raise thechemical
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wit ...
and quantum yields of nitrobenzyl-based PPGs, several beneficial modifications have been identified. The largest increase in quantum yield and reaction rate can be achieved through substitution at the benzylic carbon. However, potential substitutions must leave one hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen cons ...
so the photodegradation can proceeded uninhibited.
 Additional modifications have targeted the
Additional modifications have targeted the aromatic
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to sat ...
chromophore. Specifically, multiple studies have confirmed that the use of a 2,6-dinitrobenzyl PPG increases reaction yield. Additionally, depending on the leaving group, the presence of a second nitro-group may nearly quadruple the quantum yield (e.g. ''Φ'' = 0.033 to ''Φ'' = 0.12 when releasing a carbonate at 365 nm). While one may credit the increase in efficiency to the electronic effect
An electronic effect influences the structure, reactivity, or properties of molecule but is neither a traditional bond nor a steric effect. In organic chemistry, the term stereoelectronic effect is also used to emphasize the relation between t ...
s of the second nitro group, this is not the case. Analogous systems with a 2-cyano-6-nitrobenzyl PPG exhibit similar electron-withdrawing effects, but do not provide such a large increase in efficiency. Therefore, the increase in efficiency is likely due to the increased probability of achieving the ''aci-''nitro state; with two nitro groups, an incoming photon will be twice as likely to promote the compound into an excited state.
Finally, changing the excitation wavelength of the PPG may be advantageous. For example, if two PPGs have different excitation wavelengths one group may be removed while the other is left in place. To this end, several nitrobenzyl based PPGs display additional functionality. Common modifications include the use of 2-nitroveratryl (NV) or 6-nitropiperonulmethyl (NP). Both of these modifications induced red-shifting in the compounds' absorption spectra.
Carbonyl-based PPGs
Phenacyl PPGs
 The phenacyl PPG is the archetypal example of a carbonyl-based PPG. Under this motif, the PPG is attached to the protected substrate at the αβ-carbon, and can exhibit varied photodeprotection mechanisms based on the phenacyl skeleton, substrate identify and reaction conditions. Overall, phenacyl PPGs can be used to protect
The phenacyl PPG is the archetypal example of a carbonyl-based PPG. Under this motif, the PPG is attached to the protected substrate at the αβ-carbon, and can exhibit varied photodeprotection mechanisms based on the phenacyl skeleton, substrate identify and reaction conditions. Overall, phenacyl PPGs can be used to protect sulfonate
In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non- ...
s, phosphates, carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carbox ...
s and carbamates.
As with nitrobenzyl-based PPGs, several modifications are known. For example, the 3’,5’-dimethoxybenzoin PPG (DMB) contains a 3,5-dimethoxyphenyl substituent on the carbonyl's α-carbon. Under certain conditions, DMB has exhibited quantum yields as high as 0.64. Additionally, the p-hydroxyphenacyl PPG (''p''HP) has been designed to react through a photo-Favorskii rearrangement. This mechanism yields the carboxylic acid as the exclusive photoproduct; the key benefit of the ''p''HP PPG is the lack of secondary photoreactions and the significantly different UV absorption profiles of the products and reactants. While the quantum yield of the ''p''-hydroxyphenacyl PPG is generally in the 0.1-0.4 range, it can increase to near unity when releasing a good leaving group such as a tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a tolyl group, –, joined to a sulfonyl group, ––, with the open valence on ...
ate. The photoextrusion of the leaving group from the ''p''HP PPG is so effective, that it also releases even poor nucleofuges such as amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such ...
(with the quantum yield in the 0.01-0.5 range, and dependent on solution pH). The Additionally, photorelease occurs on the nanosecond timeframe, with ''krelease'' > 108 s−1.
The ''o''-hydroxyphenacyl PPG has been introduced as an alternative with absorption band shifted closer towards the visible region, however it has slightly lower quantum yields of deprotection (generally 0.1-0.3) due to excited state proton transfer available as an alternative deactivation pathway.
The phenacyl moiety itself contains one chiral carbon atom in the backbone. The protected group (leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
) is not directly attached to this chiral carbon atom, however has been shown to be able to work as a chiral auxiliary directing approach of a diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature ...
to a dienophile in a stereoselective thermal Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexe ...
. The auxiliary is then removed simply upon irradiation with UV light.
Photoenolization through γ-hydrogen abstraction
Another family of carbonyl-based PPGs exists that is structurally like the phenacyl motif, but which reacts through a separate mechanism. As the name suggests, these PPGs react through abstraction of the carbonyl's γ-hydrogen. The compound is then able to undergo a photoenolization, which is mechanistically like aketo-enol tautomerization
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (). The ...
. From the enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (). The te ...
form, the compound can finally undergo a ground-state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. In ...
transformation that releases the substrate. The quantum yield of this mechanism directly corresponds to the ability of the protected substrate to be a good leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
. For good leaving groups, the rate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
is either hydrogen abstraction
In chemistry, a hydrogen atom abstraction or hydrogen atom transfer (HAT) is any chemical reaction in which a hydrogen free radical (neutral hydrogen atom) is abstracted from a substrate according to the general equation:
:X^\bullet + H-Y -> X-H ...
or isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautome ...
; however, if the substrate is a poor leaving group, release is the rate-determining step.
Benzyl-based PPGs
 Barltrop and Schofield first demonstrated the use of a benzyl-based PPG, structural variations have focused on substitution to the
Barltrop and Schofield first demonstrated the use of a benzyl-based PPG, structural variations have focused on substitution to the benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
ring, as well as extension of the aromatic core. For example, insertion of a m,m’-dimethoxy substituent was shown to increase the chemical yield ~75% due to what has been termed the “excited state ''meta'' effect.” However, this substitution is only able to release good leaving groups such as carbamates and carboxylates. Additionally, the addition of an ''o''-hydroxy group enables the release of alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s, phenols and carboxylic acids due to the proximity of the phenolic hydroxy to the benzylic leaving group. Finally, the carbon skeleton has been expanded to include PPGs based on naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromat ...
, anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is ...
, phenanthrene
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics and pesticides, ...
, pyrene
Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is . This yellow solid is the smallest peri-fused PAH (one where the rings are fused through mor ...
and perylene
Perylene or perilene is a polycyclic aromatic hydrocarbon with the chemical formula C20H12, occurring as a brown solid. It or its derivatives may be carcinogenic, and it is considered to be a hazardous pollutant. In cell membrane cytochemistry ...
cores, resulting in varied chemical and quantum yields, as well as irradiation wavelengths and times.
Applications
Use in total synthesis
Despite their many advantages, the use of PPGs in total syntheses are relatively rare. Nevertheless, PPGs’ "orthogonality" to common synthetic reagents, as well as the possibility of conducting a "traceless reagent process", has proven useful innatural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical sy ...
synthesis. Two examples include the syntheses of ent-Fumiquinazoline and (-)- diazonamide A. The syntheses required irradiation at 254 and 300 nm, respectively.


Photocaging
 Protecting a substrate with a PPG is commonly referred to as "photocaging." This term is especially popular in biological systems. For example, Ly ''et al.'' developed a ''p''-iodobenzoate-based photocaged reagent, which would experience a homolytic photoclevage of the C-I bond. They found that the reaction could occur with excellent yields, and with a
Protecting a substrate with a PPG is commonly referred to as "photocaging." This term is especially popular in biological systems. For example, Ly ''et al.'' developed a ''p''-iodobenzoate-based photocaged reagent, which would experience a homolytic photoclevage of the C-I bond. They found that the reaction could occur with excellent yields, and with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of 2.5 minutes when a 15 W 254 nm light source was used. The resulting biomolecular radicals are necessary in many enzymatic
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. ...
processes. As a second example, researchers synthesized a cycloprene-modified glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
photocaged with a 2-nitroveratrol-based PPG. As it is an excitatory amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neur ...
, the aim was to develop a bioorthagonal probe for glutamate ''in vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and ...
''. In a final example, Venkatesh ''et al.'' demonstrated the use of a PPG-based photocaged therapeutic. Their prodrug, which released one equivalent of caffeic acid
Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, o ...
and chlorambucil
Chlorambucil, sold under the brand name Leukeran among others, is a chemotherapy medication used to treat chronic lymphocytic leukemia (CLL), Hodgkin lymphoma, and non-Hodgkin lymphoma. For CLL it is a preferred treatment. It is given by mouth.
...
upon phototriggering, showed reasonable biocompatibility
Biocompatibility is related to the behavior of biomaterials in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoing de ...
, cellular uptake and photoregulared drug release ''in vitro
''In vitro'' (meaning in glass, or ''in the glass'') studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in biology and ...
''.
Photoresists
During the 1980s,AT&T Bell Laboratories
Nokia Bell Labs, originally named Bell Telephone Laboratories (1925–1984),
then AT&T Bell Laboratories (1984–1996)
and Bell Labs Innovations (1996–2007),
is an American industrial Research and development, research and scientific developm ...
explored the use of nitrobenzyl-based PPGs as photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronic industry.
...
s. Over the course of the decade, they developed a deep UV positive-tone photoresist where the protected substrate was added to a copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are some ...
of poly(methyl methacrylate)
Poly(methyl methacrylate) (PMMA) belongs to a group of materials called engineering plastics. It is a transparent thermoplastic. PMMA is also known as acrylic, acrylic glass, as well as by the trade names and brands Crylux, Plexiglas, Acryli ...
and poly(methacrylic acid)
Poly(methacrylic acid) (PMAA) is a polymer made from methacrylic acid (preferred IUPAC name, 2-methylprop-2-enoic acid), which is a carboxylic acid. It is often available as its sodium salt, poly(methacrylic acid) sodium salt. The monomer is a ...
. Initially, the blend was insoluble. However, upon exposure to 260 ± 20 nm light, the PPG would be removed yielding 2-nitrosobenzaldehyde and a carboxylic acid that was soluble in aqueous base.
Surface modification
 When
When covalently
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
attached to a surface, PPGs do not exhibit any surface-induced properties (i.e. they behave like PPGs in solution, and do not exhibit any new properties because of their proximity to a surface). Consequently, PPGs can be patterned on a surface and removed in manner analogous to lithography
Lithography () is a planographic method of printing originally based on the immiscibility of oil and water. The printing is from a stone ( lithographic limestone) or a metal plate with a smooth surface. It was invented in 1796 by the German ...
to create a multifunctionalized surface. This process was first reported by Solas in 1991; protected nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecul ...
s were attached to a surface and spatially-resolved single stranded polynucleotide
A polynucleotide molecule is a biopolymer composed of 13 or more nucleotide monomers covalently bonded in a chain. DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are examples of polynucleotides with distinct biological function. The pref ...
s were generated in a step-wise “grafting from” method. In separate studies, there have been multiple reports of using PPGs to enable the selective separation of blocks within block-copolymers to expose fresh surfaces. Furthermore, this surface patterning method has since been extended to proteins. Caged etching agents (such as hydrogen fluoride protected with 4-hydroxyphenacyl) allows to etch only surfaces exposed to light.
Gels
Various PPGs, often featuring the 2-nitrobenzyl motif, have been used to generate numerous gels. In one example, researchers incorporated PPGs into asilica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is o ...
-based sol-gel. In a second example, a hydrogel was synthesized to include protected Ca2+ ions. Finally, PPGs have been utilized to cross-link numerous photodegradable polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and ...
s, which have featured linear, multi-dimensional network, dendrimer, and branched structures.
References
{{Reflist, 30em Protecting groups Photochemistry